Preprint
Review
Exploring the Potential of Nanotechnology in Cosmetics: Incorporating Natural Ingredients for Enhanced Skin Benefits
Altmetrics
Downloads
278
Views
171
Comments
0
This version is not peer-reviewed
Abstract
This article presents a comprehensive overview of the utilisation of nanotechnology in the cosmetics industry, with a specific focus on developing nanoparticles for the efficient delivery of active ingredients through skin penetration. The review delves into incorporating natural ingredients in cosmetics, exploring their therapeutic properties and the advantages and limitations associated with their use. Furthermore, the article examines the benefits of integrating natural ingredients into skincare products to enhance skin conditions, highlighting the application of nonfibrous technology in areas such as UV protection, anti-ageing effects, moisturisation improvement, and wound healing. Ultimately, the article emphasises the immense potential of nanotechnology to create innovative opportunities and positive societal impacts across various industries.
Keywords:
Submitted:
01 November 2023
Posted:
09 November 2023
You are already at the latest version
Alerts
This version is not peer-reviewed
Submitted:
01 November 2023
Posted:
09 November 2023
You are already at the latest version
Alerts
Abstract
This article presents a comprehensive overview of the utilisation of nanotechnology in the cosmetics industry, with a specific focus on developing nanoparticles for the efficient delivery of active ingredients through skin penetration. The review delves into incorporating natural ingredients in cosmetics, exploring their therapeutic properties and the advantages and limitations associated with their use. Furthermore, the article examines the benefits of integrating natural ingredients into skincare products to enhance skin conditions, highlighting the application of nonfibrous technology in areas such as UV protection, anti-ageing effects, moisturisation improvement, and wound healing. Ultimately, the article emphasises the immense potential of nanotechnology to create innovative opportunities and positive societal impacts across various industries.
Keywords:
Subject: Chemistry and Materials Science - Biomaterials
1. Introduction
Nanotechnology is a rapidly growing field in the cosmetics industry that involves manipulating materials at the nanoscale to create nanoparticles that can penetrate the skin and deliver active ingredients more effectively [1]. Nanotechnology involves studying substances at the molecular and atomic levels, focusing on objects and structures calibrated on a nanometre scale, which is one billionth of a meter (10-9 m) [2]. For comparison, the diameter of an influenza virus is 100 nm, whereas the thickness of human hair is approximately 100 µm [3].
Nanotechnology in cosmetic products has led to innovative products with improved performances [4]. Natural products are commonly used in cosmetics because of their therapeutic properties and minimal side effects [5]. Despite this, the safety of nanoparticles in cosmetic products is a concern, and further research is needed to fully understand their impact on human health and the environment [6].
Herbal cosmetics offer advantages such as better patient tolerance [7], minimal side effects [8], renewable sources of medication [7], extensive availability [9], and cost-effectiveness [10]. Nevertheless, it also has disadvantages, such as slower growth in demand, testing difficulties and limited availability, strict manufacturing procedures and a lack of standardisation in ingredients and techniques [11]. Skin is the primary barrier that shields the body from various free radicals [12]. Various sources produce free radicals, such as UV rays, dust, chemicals, and air pollution [13].
People of all ages seek premium skincare products for flawless, youthful skin. The quality and density of extracellular matrices and the provision of cells to connective tissues influence the concept of ideal skin [14]. Skin conditions such as acne, abnormal pigmentation, and xerosis can indicate skin pathology [15]. Nutritional deficiencies can cause skin lesions; however, combining cosmetic skin care products and over-the-counter (OTC) treatments can help individuals improve their skin health and appearance [16]. By following a regimen that includes both products, consumers can rebuild their skin and achieve a more beautiful complexion [17]. Natural ingredients are substances derived from natural sources, such as plants or minerals, without synthetic or artificial additives, such as coconut oil, shea butter, or lavender essential oil [10]. Incorporating natural ingredients into skin care products improves skin conditions [18]. Nanotechnology is used in various ways in skincare products to provide benefits such as UV protection [19], anti-ageing effects [20], improved moisturisation [21], and wound healing [10].
Nanomaterials are increasingly being used in various industries, including cosmetics [22], pharmaceuticals [23], and dermatology [24]. In cosmetics, nanomaterials are used as hair conditioners [25], serums [26], moisturisers [27], shampoos [28] for damaged hair, skin-lightening creams, and anti-ageing creams [4].
Nanofibrous technology allows for the encapsulation of nanoparticles, which then act as a drug delivery substrate, allowing the active components to reach deeper layers of the skin, where they can have the most effect [15]. For these reasons, nanofibrous technology is gaining popularity in cosmetics and medicine.
This study endeavours to investigate the potential of nanotechnology in the cosmetics field by incorporating natural ingredients to amplify skin benefits. By filling the existing research gap, it aims to elucidate the effective utilisation of nanotechnology in seamlessly integrating natural ingredients into cosmetic formulations. This research offers novel insights into the application of nanotechnology and presents a fresh perspective on harnessing the potential of natural ingredients in skincare. The findings of this study hold promise for advancing our understanding of how nanotechnology can revolutionise the use of natural ingredients in skincare and pave the way for innovative approaches in cosmetic product development.
2. Natural active ingredients
Table 1 presents a compilation of scientifically backed active ingredients derived from natural sources, curated for their potential effectiveness in addressing various skin types. Specifically, for individuals with dry skin, the following ingredients are recommended: aloe vera (Aloe barbadensis), chamomile (Matricaria chamomilla), calendula (Calendula officinalis), lavender (Lavandula angustifolia), coconut oil (Cocos nucifera), jojoba oil (Simmondsia Chinensis), shea butter (Butyrospermum parkii), olive oil (Olea europaea), rosehip oil (Rosa canina), and witch hazel (Hamamelis virginiana). These ingredients have been identified for their potential to provide soothing, moisturising, and hydrating effects, offering a natural approach to alleviate dryness and promote improved skin health [29]. Furthermore, cosmeceuticals have the ability to regulate the distribution of their active ingredients by forming a thin film on the skin, facilitating targeted and precise delivery [8]. This controlled release mechanism enables the recommended ingredients for normal, oily, and combination skin types, such as niacinamide, vitamin C, hyaluronic acid, green tea extract, salicylic acid, tea tree oil, zinc, witch hazel, alpha hydroxy acids, jojoba oil, to exert their beneficial effects in specific areas as needed. By incorporating such advanced delivery systems, cosmeceuticals can optimise the efficacy of these ingredients, ensuring their effective penetration into the skin and enhancing their desired outcomes [30].
UV radiation exposure accounts for approximately 90% of skin ageing [48]. The skin’s ageing process can also be influenced by lifestyle factors such as smoking and sleeping habits, exposure to pollution and poor diet [49]. The first signs of skin ageing include dryness, wrinkling and loss of elasticity [50]. In the pursuit of preventing or decelerating the onset of early signs of skin ageing, various modern interventions have emerged [51]. While traditional topical formulations, including emulsions, suspensions, solutions, gels, powders, and aerosols, continue to be widely employed for delivering active ingredients to the skin, advancements in scientific research have opened up new avenues for innovative approaches [52]. These novel interventions aim to enhance the efficacy of anti-ageing treatments by harnessing cutting-edge technologies, such as nanotechnology, microencapsulation, and targeted delivery systems. By leveraging these advancements, scientists and researchers can devise formulations that optimise active ingredients’ bioavailability, stability, and controlled release, thereby maximising their impact on skin health and rejuvenation [53,54,55]. Traditional topical skin care formulations may have limitations that affect their safety and efficacy [56]. Researchers have developed various nanomaterials to overcome these limitations to facilitate drug delivery [57]. Using nanomaterials to develop skin care products is an ongoing process in the healthcare and cosmetics industries, potentially creating new opportunities and positive impacts on society and various industries [58]. Nano-sized drug delivery systems are being studied to improve the delivery of active pharmaceutical ingredients (APIs) in nano-products such as cosmetics and pharmaceuticals [59]. The skin is a barrier, and the administration of APIs can be challenging because of the complex physiological layers with different polarities [60]. Various active ingredients in cosmetic products can prevent, delay and treat skin ageing [61]. Nanofibers have been studied as potential solutions to address the challenges of transdermal drug delivery [62]. Skin care products that use nanotechnology, including nano-products, have demonstrated promising results in delivering active ingredients into the skin [7,9,43,53].
3. Skin structure
The epidermis, dermis, and subcutaneous layers constitute the skin’s largest organ. Figure 1 shows the structure of the skin. The epidermis regenerates continually and contains keratinocytes and other cells that divide at the base and move toward the surface [63]. As part of the natural process, skin cells undergo apoptosis and contribute to forming the outermost layer of the epidermis, known as the stratum corneum [64]. Melanocytes, specialised cells in the skin, produce melanin, a pigment responsible for skin colouration and a protective shield against UV radiation [65]. The dermis houses sweat glands, which release perspiration through ducts, enabling the body to regulate its temperature [66].
Additionally, dermal hair follicles play a role in temperature regulation [67]. These cellular components and mechanisms contribute to maintaining the functionality and protection of the skin, ensuring its vital role in thermoregulation [68]. Sebaceous glands generate sebum, an oil that protects hair from bacteria and dust and creates the skin surface layer with perspiration [13,14].
Figure 1.
Anatomy of the Skin [69].
Figure 1.
Anatomy of the Skin [69].

3.1. Keratinocytes
The epidermis, the outermost layer of the skin, consists mainly of keratinocytes and acts as a protective barrier, maintaining moisture levels and shielding the skin from external factors [70]. It has a layered structure, with the youngest cells in the basal layer and the oldest cells on the surface [71]. Stem cells in the basal layer continuously regenerate and renew the skin, while the granular layer synthesises lipids, proteins, and carbohydrates, forming multi-coloured granules [63]. The outermost layer of flattened and inactive cells is called the stratum corneum (SC) [72].
Skin tone can be dull or tired, and darker-skinned people can describe it as "ashy" due to this buildup caused by a lack of natural enzymes that break down dead skin cells [73]. Keratinocytes can be converted into cancerous cells by losing their ability to survive programmed cell death, or a process called apoptosis squamous cell carcinoma (SCC) or basal cell carcinoma (BCC), depending on the location of the affected cells, which reproduce and produce multiple copies of themselves, resulting in the formation of squamous cell carcinoma (SCC) or basal cell carcinoma (BCC) is more commonly observed in individuals who have a history of significant sun exposure cancers of these types of cancer are usually treated with surgery, which can result in scarring antioxidants and retinoids, according to some studies, may help prevent the formation of SCCs and BCCs. [74,75].
3.2. Fibroblasts
Fibroblasts are located in the middle layer of the skin over the muscle and fat layers [76]. Fibroblasts are vital cells in skin ageing because they secrete collagen, elastin, and hyaluronic acid (HA), which affect the appearance of skin collagen, elastin, and HA, the skin’s thickness, volume, elasticity, and strength are dependent on fibroblasts. After all, they contain the cellular organelles that produce collagen, elastin, and HA, which are essential skin components [77,78].
3.2.1. Collagen
Collagen is the primary component of connective tissue and is a group of proteins characterised by their unique triple-helix structure [79]. The dermis contains 11 out of 18 types of collagen [80]. The most prevalent protein found in humans is Type I collagen, whereas Type I, III, and VII collagen are the most significant types of collagen in the skin [81]. Fibroblasts produce collagen in a pre-formed state that acts as a scaffold or structure, providing structural support and strength to the skin. As skin ages, it contains lower amounts of Type I and Type III collagen than young skin. [82]. laser treatments, light therapy, and injectable hyaluronic acid (HA) products have been shown to increase collagen production in the skin [83,84,85,86].
3.2.2. Elastin
Elastin is a crucial element of the extracellular matrix of connective tissue and consists of two primary components: fibrillin and tropoelastin [87]. Fibroblasts separate fibrillin and tropoelastin components, and the skin must assemble them to form mature elastin fibrils that provide elasticity to the skin [88]. After puberty, the skin has trouble producing functional elastin; therefore, ageing skin sags and loses its elasticity [88]. Aged skin exhibits reduced levels of functional elastin compared to younger skin. Presently, there are no topical or injectable medications that effectively stimulate the synthesis of functional elastin in the skin [89,90].
3.2.3. Hyaluronic Acid
Hyaluronic acid (HA) is a glycosaminoglycan abundantly present in the dermis [91]. It is a sugar molecule capable of binding to water up to 1,000 times its weight, aiding the skin’s ability to attract and retain moisture, thereby maintaining its volume [92]. HA plays vital roles in cell development, membrane receptor function, and cell adhesion while also crucial for skin hydration [21]. With age, the levels of HA in the joints and skin decrease, resulting in a decline in skin plumpness [93]. HA is the active component in dermal fillers like Restylane and Juvéderm [94]. The study conducted by Poetschke et al. [95] aimed to assess the efficacy of the daily application of hyaluronic acid-containing anti-wrinkle creams on wrinkle depth, skin tightness, and elasticity. The findings revealed significant improvements in wrinkle depth and skin tightness following consistent use for over 3 months. However, due to limitations in the study design, conclusive evidence regarding the efficacy of hyaluronic acid could not be established.
4. Encapsulation methods
Encapsulation in cosmetics involves the encapsulation of active ingredients within small particles or spheres composed of polymers, lipids, or other materials, serving to protect the active ingredient from degradation or evaporation and enabling controlled release over time, thereby enhancing the stability and efficacy of cosmetic products and facilitating the delivery of vitamins, antioxidants, and peptides to the skin [95].
5.1. Polymer-based nanoparticles
Polymeric nanoparticles are small and have unique properties that make them useful for various applications, including drug delivery. They can improve drug stability, provide controlled release, enhance drug-tissue interaction, and reduce adverse effects by improving the therapeutic index, making them suitable for various fields [20]. Polymer-based nanoparticles are spherical nanocarriers that are available in nanocapsules and nanospheres. The former has a hollow core surrounded by a polymeric layer, while the latter has a polymeric matrix that extends from the body to the surface. Molecule location is determined by its chemical structure; in other cases, the active molecule’s location is determined by its chemical structure; in other cases, it can be adsorbed or grafted onto the particles’ surface. [10,11,23]. Chitosan, alginate, and proteins like albumin are some of the most extensively studied natural polymers in polymeric nanoparticles for drug delivery [50]. Several synthetic polymers have been extensively studied to produce polymeric nanoparticles, with polylactide-polyglycolide, polylactide, polycaprolactones, and polyacrylates being some of the most widely explored [31]. Polymer-based nanoparticles have attracted much attention in various applications, particularly drug delivery systems. Nanoparticles have unique properties that enhance drug stability, controlled release, drug-tissue interaction, and reduced adverse effects, thereby raising the therapeutic index [96]. In addition to their applications in drug delivery systems, polymer-based nanoparticles have garnered significant attention in the cosmetic industry [97]. Provide promising opportunities for improving the quality and performance of cosmetic products. Cosmetics can achieve improved stability, controlled release, and targeted delivery of beneficial compounds to the skin by incorporating active ingredients into polymer-based nanoparticles [98].
Figure 2.
Schematic representation of the structure of nanocapsules and nanospheres (arrow stands for the presence of drug/bioactive within the nanoparticles).
Figure 2.
Schematic representation of the structure of nanocapsules and nanospheres (arrow stands for the presence of drug/bioactive within the nanoparticles).

5.2. Nanoprecipitation method
Nanoprecipitation is a rapid and reproducible technique that produces submicron particles with a well-defined size distribution in a single step [99]. The initial publication on this technique was authored by Fessi et al. [20]. Nanoprecipitation occurs when an organic solution containing a polymer not soluble in water is mixed with water (the non-solvent phase) during nanoprecipitation [100]. Combine, but the resultant mixture is a poor solvent for the chosen polymer, causing it to precipitate immediately through solvent diffusion, a technique known as solvent diffusion [13]. Nanocapsules are made using the same technique, except oil is included in the organic phase to form an internal oily core [9]. Nanoprecipitation has several advantages, including simplicity, speed, consistency, and the ability to scale up the process [1].
In addition, the nanoprecipitation technique eliminates the need for a stabiliser or high-energy production for charged polymers. A significant drawback of this method is the low loading efficiency for hydrophilic drugs due to the hydrophobic nature of the nanoparticle matrix [42]. The nanoparticle matrix’s water-repellent properties can hinder the absorption of water-soluble drugs in the nanoprecipitation technique [100]. Nevertheless, several strategies have been proposed to enhance the incorporation of these substances [46].
5.3. Simple emulsion evaporation method
Vanderhoff et al. introduced a straightforward emulsification evaporation technique for producing polymeric nanoparticles.[45]. In this technique, pharmaceutical drugs and polymers are initially dissolved in a volatile organic solvent that is insoluble in water. Subsequently, nanoparticles are formed through the process, yielding a homogeneous dispersion of drug-polymer particles [101]. The emulsion solvent evaporation technique produces nanoparticles, wherein an organic polymer solution is emulsified with an aqueous phase containing a stabiliser [102]. The polymer precipitates and nanoparticles are formed by applying high-shear stress and stirring, resulting in a well-dispersed system [103]. Hydrophobic drugs have a high encapsulation rate but can result in polydisperse particles and are less effective than hydrophilic drugs [20]. Ruiz et al. investigated the impact of sonication parameters on the preparation of polymeric nanoparticles using the emulsion-solvent evaporation technique. The study optimised the conditions for obtaining polymeric nanoparticles with polylactic acid (PLA), achieving particle sizes ranging from 110 to 240 nm and PDI values between 0.09 and 0.32. Power was found to have the most significant influence on particle size and PDI, followed by sonication time and the number of sonication cycles—the optimised PLA nanoparticles indicated excellent size homogeneity and potential for developing targeted nano formulations [104].
Figure 3.
Preparation of nanocapsules by emulsion diffusion method.

5.4. Double emulsion evaporation method
The double emulsion evaporation method, also known as the water-in-oil-in-water (W/O/W) method, is a widely used encapsulation technique for the preparation of nanoparticles. It is beneficial for encapsulating hydrophilic (water-soluble) substances, such as drugs and proteins, within a hydrophobic nanoparticle matrix. [16]. Encapsulation in the double emulsification evaporation method involves homogenising an oily phase (O) that typically consists of a polymer solution dissolved in an organic solvent containing the required hydrophobic drug, with an aqueous phase (W1) containing a hydrophilic drug. In the second step, the first W1/O emulsion is emulsified in another aqueous phase (W2) containing a suitable stabiliser using high-shear homogenisation or low-power sonication to create a double emulsion (W1/O/W2). While this approach can encapsulate both hydrophilic and hydrophobic drugs, it has several drawbacks, including low colloidal stability of the resulting particles, high shear stirring, which can lead to emulsion fragmentation, foam formation, and polydisperse particles more significant than a micron in size [40]. Iqbal et al. demonstrated that by optimising process parameters, such as ultrasound exposure time, amplitude, outer aqueous phase volume, PCL content, and PVA concentration, they successfully prepared biodegradable PCL nanoparticles with reduced particle size and spherical morphology using the double emulsion solvent evaporation method combined with power ultrasound [105].
Figure 4.
The schematic diagram for the double emulsion solvent evaporation method.

5.5. Ionic gelation
Ionic gelation is a widely employed technique for the formulation of polymeric nanoparticles or microparticles, characterised by crosslinking a water-soluble polymer utilising a compatible ionic crosslinking agent [20]. This method involves establishing ionic interactions between the polymer chains and the crosslinking agent, leading to forming a gel-like network structure [106]. Through careful control of the reaction conditions, such as the selection of appropriate polymers and crosslinkers, as well as their concentrations and reaction parameters, the resulting particles’ size, shape, and stability can be tailored to meet specific requirements [107]. Giri et al., ionotropic gelation involve the formation of nanoparticles through the interaction between polyelectrolytes and counter ions, where chitosan polysaccharide is dissolved in an aqueous acidic solution to obtain the cation of chitosan. This cationic solution is subsequently added drop-wise to a polyanionic tripolyphosphate solution under continuous stirring, resulting in ionic gelation and precipitation of chitosan as spherical particles [108].
Figure 5.
A schematic illustration of ionic gelation reaction.

5.6. Supercritical fluid method
The methods frequently require toxic solvents and surfactants that can pose risks to the environment and physiological systems [20]. As a result, current research have been focused on exploring safer techniques to produce carriers for various applications. One such approach that has piqued the interest of many researchers is the use of supercritical fluids, considered environmentally friendly solvents [39]. Revercheon et al. emphasised the application of supercritical fluid-based techniques for synthesising a wide range of nanostructures, including nanofibers, nanotubes, nanowires, nanoparticles, and other nano-architectures [109]. Byrappa et al. investigated the versatile capabilities of supercritical fluids (SCFs) in the fabrication of advanced nanomaterials, encompassing carbon nanotubes, fullerenes, magnetic particles, quantum dots, phosphors, nanocomposites (such as peptide/hydroxyapatite), and gold nanoshells. These nanomaterials hold significant potential for various biomedical applications, including drug delivery, imaging, sensing, and cancer theranostics [110]. Supercritical fluid technology emerges as a promising approach in the formulation of drug carriers, offering notable advantages such as utilising environmentally friendly solvents [111]. Supercritical CO2 is a commonly used fluid used in the supercritical antisolvent process to instantaneously precipitate particles from a drug and polymer dissolved in a liquid solvent [112]. However, this technique has some limitations, including the need for specialised equipment and higher costs for routine use [28].
Figure 6.
Flow diagram of supercritical fluid extraction system.

6. Electrospinning for encapsulated active ingredients
Electrospinning produces ultrafine fibres characterised by a high surface area to volume ratio, making them well-suited as encapsulation materials [113]. These fibres can serve as a delivery system for controlled release applications or as a scaffold for tissue engineering purposes by incorporating active ingredients into the polymer solution prior to electrospinning [114]. Conversely, electrospinning involves the creation of nanofibers by subjecting polymer solutions or melts to an electric charge [115]. Both methodologies can be employed for delivering active ingredients to the skin and may be combined to enhance efficacy and achieve targeted delivery [116]. Figure 7 illustrates the encapsulation technique, wherein active ingredients are encapsulated within a protective outer shell, followed by the electrospinning of the prepared solution.
6.1. Electrospinning methods
Electrospinning is a scientifically established process that utilises an electric field to induce the transformation of a charged polymer solution or melt into finely spun fibres [117]. The process commences with preparing a polymer solution, which is subsequently loaded into a syringe or spinneret apparatus [118]. The polymer solution is carefully extruded through a needle or spinneret orifice, subsequently experiencing the influence of a high-voltage electric field [117]. This causes the solution to become charged and form a Taylor cone at the tip of the needle [113]. As the electric field strength increases, the surface tension of the solution is overcome, and a charged jet is ejected from the cone [119]. The jet undergoes a whipping motion as it travels towards a grounded collector, and as it dries, it solidifies into a continuous nanofiber [120]. The diameter of the fibres can be controlled by adjusting the concentration of the polymer solution [121], the flow rate [122], and the strength of the electric field [118]. The electrospinning process is versatile and can be used with various polymers, including synthetic and natural materials. It has numerous applications in tissue engineering [123], drug delivery [124], filtration [125], energy storage [126], and sensors [3]. Several electrospinning approaches that can be used to encapsulate active ingredients and nanofiber productions are given in Figure 8.
Nanofiber encapsulation is an attractive approach for incorporating drugs into polymer carriers; it allows for precise control over the size and morphology of the resulting fibres. Figure 9 shows various cross-sections of electrospun fibres form that can be used to encapsulate active ingredients.
6.1.1. Single Fluid Electrospinning
- Blend Electrospinning
This approach involves dissolving the drug and the polymer carrier in a suitable solvent to create a homogenous spinning solution. Electrospinning this solution can achieve a wide range of drug release profiles, ranging from very rapid (within seconds) to sustained release over weeks or even months. The choice of solvent, polymer and processing conditions can be optimised to achieve the desired release profile for a particular drug and delivery application [47]. The main weakness of this approach is the commonly observed burst release phenomenon [30].
- Emulsion Electrospinning
Emulsion electrospinning is a scientifically established technique to fabricate core-shell nanofibers, enabling the encapsulation of growth factors, proteins, and drugs within the core region to enhance drug stability and improve bioavailability [127]. Forming a stable emulsion necessitates three crucial components: an oil phase, a water phase, and surfactants/emulsifiers, all of which influence the drug-release properties of the resulting fibres based on their respective compositions [128]. Typically, a hydrophobic polymer is dispersed in an organic solvent (oil phase), while hydrophilic compounds are dispersed in water, ensuring the desired characteristics of the emulsion for successful electrospinning. [20]. Tao et al. successfully manufactured polycaprolactone/carboxymethyl chitosan/sodium alginate fibres using emulsion electrospinning with minimal organic solvents. The resulting fibres positively impacted osteoblast viability and osteogenesis [41].
6.1.2. Multi-Fluid Electrospinning
- Multi-Jet Electrospinning
Multi-jet electrospinning can be implemented through two distinct methods: needleless and needle-based configurations [129]. This technique offers substantial advantages for large-scale nanofiber fabrication because it considerably enhances throughput. By enabling the simultaneous electrospinning of multiple jets, the production rate can be significantly increased, thereby facilitating the efficient manufacturing of nanofibers on a larger scale [130]. Moreover, it enables the preparation of multi-component fibre mats, wherein diverse populations of fibres fabricated from distinct materials are seamlessly integrated into a unified scaffold. This capability facilitates the development of complex and versatile structures with tailored properties, opening up possibilities for a wide range of applications in tissue engineering, filtration systems, and other fields [131]. This feature proves valuable in cases where the inclusion of multiple polymers in a formulation is necessary, but they cannot be dissolved within the same solution due to their incompatible solubilities. By employing the multi-component fibre mats produced through this technique, the simultaneous incorporation of diverse polymers becomes achievable, offering enhanced flexibility in designing materials with desired characteristics and functionalities [132]. The resulting fibre mat can deliver multiple drugs at varying rates, and the different fibre populations can also influence mechanical and cell adhesion properties [133]. However, there are some drawbacks to multi-jet spinning in the needle modality. The electric fields around the different needles can interact with each other, which can cause spinning to be erratic [134]. Determining the optimal needle arrangement poses a significant challenge in the electrospinning process. However, this obstacle can be overcome by employing needleless or auxiliary electrodes. These approaches contribute to the enhanced stability of the spinning process, thereby improving the overall quality and consistency of the resulting fibre mats [78].
- Side-by-Side Electrospinning
The side-by-side electrospinning technique involves extruding multiple spinning solutions through adjacent spinnerets. This method offers a notable advantage in the form of a side-by-side Janus morphology, enabling both compartments to establish physical contact with the biological microenvironment. The efficacy of this approach relies on the precise design of the spinneret and meticulous optimisation of the electrospinning parameters [135,136]. Zheng et al., in 2021, tamoxifen, a chemotherapeutic drug, was incorporated into polymer matrices of PVP and Ethyl cellulose (EC). The research findings highlighted the significance of shape, structure, and composition in the design of functional nanomaterials. By precisely manipulating these factors, it holds promise to develop novel materials with enhanced drug release profiles and other desirable properties for biomedical applications [137].
6.1.3. Coaxial/Multiaxial Electrospinning
Coaxial or multiaxial electrospinning enables the fabrication of nanofibers with a core-shell or multi-layered structure. This technique involves the simultaneous electrospinning of two or more spinning solutions through concentric or parallel spinnerets [138]. The outer layer of the fiber is formed by the polymer solution dispensed from the outer spinneret, while the inner layer (core) is formed by the solution dispensed from the inner spinneret [139]. Core-shell nanofibers offer various drug and biomolecule incorporation, enabling precise modulation of drug release rates and durations for advanced drug delivery [138]. Their structure enhances mechanical properties and biocompatibility, making them suitable for biomedical applications such as drug delivery, tissue engineering, and wound healing [140]. Coaxial electrospinning allows for precise control of small drug molecule release rates from a hydrophobic matrix and enables the encapsulation of liquids within nanofiber cores [141]. Baykara and Taylan fabricated core-shell fibres using a polyvinyl alcohol (PVA) shell and a core containing Nigella sativa seed oil, renowned for its antimicrobial properties. This core-shell structure effectively regulated the release of the oil, preventing sudden bursts of release [93]. This approach can be extended to various drugs or biomolecules that necessitate controlled release rates or encounter compatibility issues with the matrix material, offering a versatile solution for tailored drug delivery systems and overcoming formulation limitations [142]. Triaxial spinning, involving three fluids, allows for the production of multilayer nanofibers [143]. Liu et al. demonstrated the application of triaxial electrospinning to encapsulate ferulic acid within cellulose acetate nanofibers, resulting in a multilayer structure. The in vitro drug release from these fibres exhibited nearly zero-order kinetics, indicating a controlled and steady release rate. This technique can be utilised to encapsulate various drugs with varying properties, and the number of layers can be adjusted to achieve the desired release profile. Triaxial electrospinning holds promise for developing drug delivery systems with improved efficacy and reduced toxicity [144]. Quad-axial electrospinning fabricates nanofibers using four simultaneous fluids, enabling precise control over composition and functionality [145]. Quad-axial electrospinning enables the fabrication of intricate multi-layered structures with improved properties and customised release profiles, revolutionising drug delivery, tissue engineering, and biomedical applications [146]. Zhang et al. [147] utilised this approach to encapsulate the antimicrobial moxifloxacin in polycaprolactone and gelatine nanofibers with a quad-axial structure. Through the incorporation of moxifloxacin into the nanofibers, quad-axial electrospinning facilitated the achievement of controlled release kinetics over an extended duration, highlighting the adaptability of this technique in the design of drug delivery strategies. With its precise control over nanofiber composition and structure, quad-axial electrospinning presents a promising avenue for developing advanced drug delivery systems with tailored release profiles [147].
7. Applications of electrospun nanofiber-encapsulated ingredients in the cosmetic industry
Electrospinning has opened up new avenues for investigating the efficacy of nanofibers in enhancing the properties of matrix materials utilised in cosmetic applications [148]. The integration of composites offers a distinct advantage by combining the strength of reinforcement with the toughness of the matrix, resulting in exceptional properties that surpass those of conventional single materials [98]. The emergence of electrospinning techniques employing biopolymers has led to innovative nano-biocomposites, distinguished by their multifaceted nature, superior functionality, and commendable environmental sustainability, making them highly promising for future applications [149]. Table 2 summarises active ingredients electrospun into nanofibrous scaffolds for a specific skincare product, outlining their benefits for the skin. This information can aid in selecting skincare products tailored to address specific skin concerns.
In the cosmetic industry, enzymes are commonly used for their ability to improve skin texture, reduce wrinkles, and enhance skin hydration [217]; enzyme immobilisation is a well-established technique used in various fields due to its ability to improve the stability, activity, and reusability of enzymes [218]. However, using free enzymes in cosmetic formulations can be challenging due to their instability and susceptibility to degradation [219].
Neutrogena and Aveeno’s "active soy" formulations incorporate soy that has undergone laboratory modifications to eliminate its estrogenic components. Although these products utilise natural ingredients, they do not qualify for NPA certification due to their altered state, which aims to enhance their effectiveness [89].
8. Conclusions, Challenges, and Future Perspectives
The field of cosmetic applications has harnessed the potential of encapsulation and electrospinning techniques to facilitate the delivery of active ingredients. These methodologies offer notable benefits such as enhanced stability, safeguarding active ingredients, precise delivery targeting, and monitoring of release rates. Encapsulation and electrospinning have proven effective in extracting enzymes, vitamins, peptides, and plant extracts, employing natural polymers that resonate with consumers seeking eco-friendly and sustainable alternatives.
Nevertheless, challenges persist in encapsulation and electrospinning for cosmetic applications. Inconsistencies arise during the preparation and characterisation of encapsulated and electrospun products, resulting in variations in quality and efficiency. Developing cost-effective techniques becomes imperative to leverage encapsulation and electrospinning for large-scale production of high-quality cosmetic products. Incorporating natural and biodegradable polymers in these processes further enhances the sustainability of cosmetic products.
Prospectively, the adoption of encapsulation and electrospinning is poised for significant expansion, propelled by technological breakthroughs and escalating consumer demand for ecologically responsible and sustainable cosmetics. Nevertheless, assuring the safety and efficacy of these methodologies in cosmetic applications mandates incessant research endeavours and meticulous standardisation initiatives. Subsequent advancements will concentrate on refining encapsulation and electrospinning protocols, instituting stringent quality control measures, and deepening comprehension of their influence on product functionality and consumer contentment.
Abbreviations
| APIs: | Active pharmaceutical ingredients |
| BCC: | Basal Cell Carcinoma |
| BSTI | Baumann Skin Type Index |
| BSTS | Baumann Skin Type System |
| CIR: | Cosmetic Ingredient Review |
| CMCS: | carboxymethyl chitosan |
| CO2: | Carbon dioxide |
| DRNT | Dry, Resistant, Non-Pigmented, Tight skin |
| DRNW: | Dry-Resistant Non-Pigmented Wrinkled |
| DRPT | Dry, Resistant, Pigmented, Tight skin |
| DRPW: | Dry-Resistant Pigmented Wrinkled |
| DSNT | Dry, Sensitive, Non-Pigmented, Tight skin |
| DSNW | Dry, Sensitive, Non-Pigmented, Wrinkle-Prone skin |
| DSPT | Dry, Sensitive, Pigmented, Wrinkle-Prone/Tight skin |
| DSPW | Dry, Sensitive, Pigmented, Wrinkle-Prone skin |
| EC: | ethyl cellulose |
| FDA: | US Food and Drug Administration |
| HA: | Hyaluronic acid |
| INCI: | International Nomenclature of Cosmetic Ingredients |
| nm: | Nanometre |
| NMF: | natural moisturising factor |
| NPA: | Natural Products Association |
| O/W/O: | Oil-in-water-in-oil |
| O/W: | Oil-in-water |
| ORNW: | Oily, Resistant, Non-Pigmented, Wrinkle-Prone skin |
| ORPT: | Oily, Resistant, Pigmented, Tight skin |
| ORPW: | Oily, Resistant, Pigmented, Wrinkle-Prone skin |
| OSNT: | Oily, Sensitive, Non-Pigmented, Tight skin |
| OSNW: | Oily, Sensitive, Non-Pigmented, Wrinkle-Prone skin |
| OSPT: | Oily, Sensitive, Pigmented, Tight skin |
| OSPW: | Oily, Sensitive, Pigmented, Wrinkle-Prone skin |
| OTC: | Over-the-counter |
| PAR-2: | Protease-activated receptor-2 |
| PCL: | polycaprolactone |
| PEG: | Polyethylene Glycol |
| pH: | potential of hydrogen |
| PLA: | Poly (lactic acid) |
| PLA: | Polylactic acid |
| PVA: | Polyvinyl alcohol |
| PVP: | Polyvinylpyrrolidone |
| SA: | Sodium alginate |
| SC: | Stratum Corneum |
| SCC: | Squamous Cell Carcinoma |
| SLES: | Sodium lauryl ether sulphate |
| SLS: | Sodium lauryl sulphate |
| SPF: | Sun Protection Factor |
| TEWL: | Trans-epidermal Water Loss |
| UV: | Ultraviolet |
| UVA: | Ultraviolet A |
| UVB: | Ultraviolet B |
| VOC: | Volatile Organic CompoundFormun Üstü |
| W/O/W: | Water-in-oil-in-water |
| W/O: | Water-in-oil |
References
- Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari MJ, et al. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022;8:173. [CrossRef]
- Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019;25:112. [CrossRef]
- Qiao L, Han M, Gao S, Shao X, Wang X, Sun L, et al. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J Mater Chem B 2020;8:6333–51. [CrossRef]
- Salvioni L, Morelli L, Ochoa E, Labra M, Fiandra L, Palugan L, et al. The emerging role of nanotechnology in skincare. Advances in Colloid and Interface Science 2021;293:102437. [CrossRef]
- Liu J-K. Natural products in cosmetics. Nat Prod Bioprospect 2022;12:40. [CrossRef]
- Chauhan A, Chauhan C. Emerging trends of nanotechnology in beauty solutions: A review. Materials Today: Proceedings 2021. [CrossRef]
- Kaur A, Singh TG, Dhiman S, Arora S, Babbar R. NOVEL HERBS USED IN COSMETICS FOR SKIN AND HAIR CARE : A REVIEW n.d.
- Mohd-Setapar SH, John CP, Mohd-Nasir H, Azim MM, Ahmad A, Alshammari MB. Application of Nanotechnology Incorporated with Natural Ingredients in Natural Cosmetics. Cosmetics 2022;9:110. [CrossRef]
- Bowe WP, Pugliese S. Cosmetic benefits of natural ingredients. J Drugs Dermatol 2014;13:1021–5; quiz 26–7.
- Dini I, Laneri S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021;26:3921. [CrossRef]
- Costa EF, Magalhães WV, Di Stasi LC. Recent Advances in Herbal-Derived Products with Skin Anti-Aging Properties and Cosmetic Applications. Molecules 2022;27:7518. [CrossRef]
- Nguyen AV, Soulika AM. The Dynamics of the Skin’s Immune System. Int J Mol Sci 2019;20:1811. [CrossRef]
- Juliano C, Magrini GA. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018;5:19. [CrossRef]
- Rodan K, Fields K, Majewski G, Falla T. Skincare Bootcamp: The Evolving Role of Skincare. Plast Reconstr Surg Glob Open 2016;4:e1152. [CrossRef]
- Thibane VS, Ndhlala AR, Abdelgadir HA, Finnie JF, Staden JV. The cosmetic potential of plants from the Eastern Cape Province traditionally used for skincare and beauty. South African Journal of Botany 2019;122:475–83. [CrossRef]
- Hoang HT, Moon J-Y, Lee Y-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021;8:106. [CrossRef]
- Dlova NC, Hamed SH, Tsoka-Gwegweni J, Grobler A. Skin lightening practices: an epidemiological study of South African women of African and Indian ancestries. British Journal of Dermatology 2015;173:2–9. [CrossRef]
- Kumar V. Perspective of Natural Products in Skincare. PPIJ 2016;4. [CrossRef]
- He H, Li A, Li S, Tang J, Li L, Xiong L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed Pharmacother 2021;134:111161. [CrossRef]
- Ahmed IA, Mikail MA, Zamakshshari N, Abdullah A-SH. Natural anti-aging skincare: role and potential. Biogerontology 2020;21:293–310. [CrossRef]
- Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol 2007;6:75–82. [CrossRef]
- Hu X, He H. A review of cosmetic skin delivery. J Cosmet Dermatol 2021;20:2020–30. [CrossRef]
- Zeng Q, Qi X, Shi G, Zhang M, Haick H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano 2022;16:1708–33. [CrossRef]
- Antonio JR, Antônio CR, Cardeal ILS, Ballavenuto JMA, Oliveira JR. Nanotechnology in dermatology. An Bras Dermatol 2014;89:126–36. [CrossRef]
- Pereira-Silva M, Martins AM, Sousa-Oliveira I, Ribeiro HM, Veiga F, Marto J, et al. Nanomaterials in hair care and treatment. Acta Biomater 2022;142:14–35. [CrossRef]
- He M, Zhang W, Liu Z, Zhou L, Cai X, Li R, et al. The interfacial interactions of nanomaterials with human serum albumin. Anal Bioanal Chem 2022;414:4677–84. [CrossRef]
- Puglia C, Santonocito D. Cosmeceuticals: Nanotechnology-Based Strategies for the Delivery of Phytocompounds. Curr Pharm Des 2019;25:2314–22. [CrossRef]
- Santos AC, Panchal A, Rahman N, Pereira-Silva M, Pereira I, Veiga F, et al. Evolution of Hair Treatment and Care: Prospects of Nanotube-Based Formulations. Nanomaterials (Basel) 2019;9:903. [CrossRef]
- Ratz-Łyko A, Arct J, Pytkowska K. Moisturizing and Anti-inflammatory Properties of Cosmetic Formulations Containing Centella asiatica Extract. Indian J Pharm Sci 2016;78:27–33.
- Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol 2008;53:163–6. [CrossRef]
- Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Mol Med Rep 2010;3:895–901. [CrossRef]
- Preethi KC, Kuttan R. Wound healing activity of flower extract of Calendula officinalis. J Basic Clin Physiol Pharmacol 2009;20:73–9. [CrossRef]
- Kim HM, Cho SH. Lavender oil inhibits immediate-type allergic reaction in mice and rats. J Pharm Pharmacol 1999;51:221–6. [CrossRef]
- Evangelista MTP, Abad-Casintahan F, Lopez-Villafuerte L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomised, double-blind, clinical trial. Int J Dermatol 2014;53:100–8. [CrossRef]
- Pazyar N, Yaghoobi R, Ghassemi MR, Kazerouni A, Rafeie E, Jamshydian N. Jojoba in dermatology: a succinct review. G Ital Dermatol Venereol 2013;148:687–91.
- Lin T-K, Zhong L, Santiago JL. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int J Mol Sci 2017;19:70. [CrossRef]
- Villa C, Trucchi B, Gambaro R, Baldassari S. Green procedure for the preparation of scented alcohols from carbonyl compounds. Int J Cosmet Sci 2008;30:139–44. [CrossRef]
- Jose A, Mahey R, Sharma JB, Bhatla N, Saxena R, Kalaivani M, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy- randomised controlled trial. BMC Pregnancy Childbirth 2019;19:54. [CrossRef]
- Thring TS, Hili P, Naughton DP. Antioxidant and potential anti-inflammatory activity of extracts and formulations of white tea, rose, and witch hazel on primary human dermal fibroblast cells. J Inflamm (Lond) 2011;8:27. [CrossRef]
- Tang S-C, Yang J-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018;23:863. [CrossRef]
- Telang PS. Vitamin C in dermatology. Indian Dermatol Online J 2013;4:143–6. [CrossRef]
- Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging 2007;2:369–76.
- Silverberg JI, Jagdeo J, Patel M, Siegel D, Brody N. Green tea extract protects human skin fibroblasts from reactive oxygen species induced necrosis. J Drugs Dermatol 2011;10:1096–101.
- Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging 2006;1:327–48.
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol 2015;8:455–61. [CrossRef]
- Pazyar N, Yaghoobi R, Bagherani N, Kazerouni A. A review of applications of tea tree oil in dermatology. Int J Dermatol 2013;52:784–90. [CrossRef]
- Lin P-H, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J. Zinc in Wound Healing Modulation. Nutrients 2017;10:16. [CrossRef]
- Kohl E, Steinbauer J, Landthaler M, Szeimies R-M. Skin ageing. J Eur Acad Dermatol Venereol 2011;25:873–84. [CrossRef]
- Baumann L. Skin ageing and its treatment. J Pathol 2007;211:241–51. [CrossRef]
- Bellu E, Medici S, Coradduzza D, Cruciani S, Amler E, Maioli M. Nanomaterials in Skin Regeneration and Rejuvenation. International Journal of Molecular Sciences 2021;22. [CrossRef]
- Fletcher JR. Anti-aging technoscience & the biologization of cumulative inequality: Affinities in the biopolitics of successful aging. J Aging Stud 2020;55:100899. [CrossRef]
- Arora N, Agarwal S, Rayasa M. Latest Technology Advances in Cosmaceuticals. Int J Pharm Sci Drug Res 2012;4.
- Department of Pharmaceutical Technology, Faculty of Pharmacy, Istanbul Altınbaş University, Istanbul, Turkey, Otlatici G, Yegen G, Department of Pharmaceutical Technology, Faculty of Pharmacy, Istanbul Altınbaş University, Istanbul, Turkey, Gungor S, Department of Pharmaceutical Technology, Faculty of Pharmacy, Istanbul University, 34116, Istanbul, Turkey, et al. Overview on nanotechnology based cosmeceuticals to prevent skin aging. Istanbul J Pharm 2019;48:55–62. [CrossRef]
- Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. International Journal of Cosmetic Science 2008;30:87–95. [CrossRef]
- Mazur A, Holthoff E, Vadali S, Kelly T, Post SR. Cleavage of Type I Collagen by Fibroblast Activation Protein-α Enhances Class A Scavenger Receptor Mediated Macrophage Adhesion. PLoS One 2016;11:e0150287. [CrossRef]
- Mohammed YH, Moghimi HR, Yousef SA, Chandrasekaran NC, Bibi CR, Sukumar SC, et al. Efficacy, Safety and Targets in Topical and Transdermal Active and Excipient Delivery. Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin 2017:369–91. [CrossRef]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres M del P, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 2018;16:71. [CrossRef]
- Souto EB, Fernandes AR, Martins-Gomes C, Coutinho TE, Durazzo A, Lucarini M, et al. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Applied Sciences 2020;10:1594. [CrossRef]
- Mukherjee B. Nanosize drug delivery system. Curr Pharm Biotechnol 2013;14:1221. [CrossRef]
- Souto EB, Fangueiro JF, Fernandes AR, Cano A, Sanchez-Lopez E, Garcia ML, et al. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022;8:e08938. [CrossRef]
- Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol 2012;4:308–19. [CrossRef]
- Rajpoot K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr Pharm Des 2019;25:3943–59. [CrossRef]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Epidermis and Its Renewal by Stem Cells. Molecular Biology of the Cell. 4th edition, Garland Science; 2002.
- Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2013;1833:3471–80. [CrossRef]
- Brenner M, Hearing VJ. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem Photobiol 2008;84:539–49. [CrossRef]
- Baker LB. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature (Austin) 2019;6:211–59. [CrossRef]
- Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 2014;210:498–507. [CrossRef]
- Ibrahim AAE, Bagherani N, Smoller BR, Reyes-Baron C, Bagherani N. Functions of the Skin. In: Smoller B, Bagherani N, editors. Atlas of Dermatology, Dermatopathology and Venereology, Cham: Springer International Publishing; 2020, p. 1–11. [CrossRef]
- Fernandez-Carro E, Angenent M, Gracia-Cazaña T, Gilaberte Y, Alcaine C, Ciriza J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022;14:1417. [CrossRef]
- Lefèvre-Utile A, Braun C, Haftek M, Aubin F. Five Functional Aspects of the Epidermal Barrier. Int J Mol Sci 2021;22:11676. [CrossRef]
- Coulombe1 PA, Lee C-H. Defining keratin protein function in skin epithelia: Epidermolysis Bullosa Simplex and its aftermath. J Invest Dermatol 2012;132:763–75. [CrossRef]
- Layers of the Skin | SEER Training n.d. Available online: https://training.seer.cancer.gov/melanoma/anatomy/layers.html (accessed on 2 March 2023).
- Nakashima T, Yasumatsu R, Kuratomi Y, Masuda M, Kuwano T, Toh S, et al. Role of squamous cell carcinoma antigen 1 expression in the invasive potential of head and neck squamous cell carcinoma. Head & Neck 2006;28:24–30. [CrossRef]
- Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. International Journal of Dermatology 2004;43:326–35. [CrossRef]
- De Graaf YGL, Euvrard S, Bouwes Bavinck JN. Systemic and topical retinoids in the management of skin cancer in organ transplant recipients. Dermatol Surg 2004;30:656–61. [CrossRef]
- Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell 2021;184:3852–72. [CrossRef]
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair and Regeneration 2009;17:153–62. [CrossRef]
- Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. Journal of Cell Science 2004;117:667–75. [CrossRef]
- Shoulders MD, Raines RT. COLLAGEN STRUCTURE AND STABILITY. Annu Rev Biochem 2009;78:929–58. [CrossRef]
- P B, H S. Structurally distinct collagen types. Annual Review of Biochemistry 1980;49. [CrossRef]
- Di Lullo GA, Sweeney SM, Körkkö J, Ala-Kokko L, San Antonio JD. Mapping the Ligand-binding Sites and Disease-associated Mutations on the Most Abundant Protein in the Human, Type I Collagen*. Journal of Biological Chemistry 2002;277:4223–31. [CrossRef]
- Nelson BR, Majmudar G, Griffiths CE, Gillard MO, Dixon AE, Tavakkol A, et al. Clinical improvement following dermabrasion of photoaged skin correlates with synthesis of collagen I. Arch Dermatol 1994;130:1136–42.
- Skinner SM, Gage JP, Wilce PA, Shaw RM. A preliminary study of the effects of laser radiation on collagen metabolism in cell culture. Aust Dent J 1996;41:188–92. [CrossRef]
- Orringer JS, Kang S, Johnson TM, Karimipour DJ, Hamilton T, Hammerberg C, et al. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol 2004;140:1326–32. [CrossRef]
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg 2002;28:1115–9. [CrossRef]
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by crosslinked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol 2007;143:155–63. [CrossRef]
- Wilcox WR. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects,. Am J Hum Genet 2003;72:503–4.
- Baumann L, Bernstein EF, Weiss AS, Bates D, Humphrey S, Silberberg M, et al. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthet Surg J Open Forum 2021;3:ojab019. [CrossRef]
- Cosmeceuticals and Cosmetic Ingredients | AccessDermatologyDxRx | McGraw Hill Medical n.d. Available online: https://dermatology.mhmedical.com/book.aspx?bookID=2812 (accessed on 2 March 2023).
- Simionescu DT, Lu Q, Song Y, Lee J, Rosenbalm TN, Kelley C, et al. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials 2006;27:702–13. [CrossRef]
- Lierova A, Kasparova J, Filipova A, Cizkova J, Pekarova L, Korecka L, et al. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022;14:838. [CrossRef]
- Dovedytis M, Liu ZJ, Bartlett S. Hyaluronic acid and its biomedical applications: A review. Engineered Regeneration 2020;1:102–13. [CrossRef]
- Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a New Topical Nano-hyaluronic Acid in Humans. J Clin Aesthet Dermatol 2014;7:27–9.
- Ehlers E-M, Behrens P, Wünsch L, Kühnel W, Russlies M. Effects of hyaluronic acid on the morphology and proliferation of human chondrocytes in primary cell culture. Annals of Anatomy - Anatomischer Anzeiger 2001;183:13–7. [CrossRef]
- Casanova F, Santos L. Encapsulation of cosmetic active ingredients for topical application--a review. J Microencapsul 2016;33:1–17. [CrossRef]
- Begines B, Ortiz T, Pérez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F, et al. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020;10:1403. [CrossRef]
- Bahamonde-Norambuena D, Molina-Pereira A, Muñoz M, Zepeda K, Vilos C. Polymeric Nanoparticles in Dermocosmetic. International Journal of Morphology 2015;33:1563–8. [CrossRef]
- Fytianos G, Rahdar A, Kyzas GZ. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials (Basel) 2020;10:979. [CrossRef]
- Martínez Rivas CJ, Tarhini M, Badri W, Miladi K, Greige-Gerges H, Nazari QA, et al. Nanoprecipitation process: From encapsulation to drug delivery. International Journal of Pharmaceutics 2017;532:66–81. [CrossRef]
- Salatin S, Barar J, Barzegar-Jalali M, Adibkia K, Kiafar F, Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res Pharm Sci 2017;12:1–14. [CrossRef]
- Zhang Y, Chan HF, Leong KW. Advanced Materials and Processing for Drug Delivery: The Past and the Future. Adv Drug Deliv Rev 2013;65:104–20. [CrossRef]
- Pulingam T, Foroozandeh P, Chuah J-A, Sudesh K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022;12:576. [CrossRef]
- Morán D, Gutiérrez G, Blanco-López MC, Marefati A, Rayner M, Matos M. Synthesis of Starch Nanoparticles and Their Applications for Bioactive Compound Encapsulation. Applied Sciences 2021;11:4547. [CrossRef]
- Ruiz E, Orozco VH, Hoyos LM, Giraldo LF. Study of sonication parameters on PLA nanoparticles preparation by simple emulsion-evaporation solvent technique. European Polymer Journal 2022;173:111307. [CrossRef]
- Iqbal M, Valour J-P, Fessi H, Elaissari A. Preparation of biodegradable PCL particles via double emulsion evaporation method using ultrasound technique. Colloid and Polymer Science 2015;3:861–73. [CrossRef]
- Merino D, Casalongué C, Alvarez V. Polysaccharides as Eco-Nanomaterials for Agricultural Applications. Handbook of Ecomaterials, 2017. [CrossRef]
- Lee H, An S, Kim S, Jeon B, Kim M, Kim IS. Readily Functionalizable and Stabilizable Polymeric Particles with Controlled Size and Morphology by Electrospray. Sci Rep 2018;8:15725. [CrossRef]
- Giri TK. Nanoarchitectonics for Smart Delivery and Drug Targeting. William Andrew 2016:119–41.
- Reverchon E, Adami R. Nanomaterials and supercritical fluids. The Journal of Supercritical Fluids 2006;37:1–22.
- Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology - towards biomedical applications. Adv Drug Deliv Rev 2008;60:299–327. [CrossRef]
- Girotra P, Singh S, Nagpal K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharmaceutical Development and Technology 2012;18. [CrossRef]
- Liu G, Li J, Deng S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multi-component Systems. Pharmaceutics 2021;13:475. [CrossRef]
- Barhoum A, Bechelany M, Hamdy Makhlouf AS. Handbook of Nanofibers. 2019. [CrossRef]
- Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater 2010;11:014108. [CrossRef]
- Garg K, Bowlin G. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011;5:13403. [CrossRef]
- Zaid Alkilani A, McCrudden MTC, Donnelly RF. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015;7:438–70. [CrossRef]
- Al-Hazeem NZA, Al-Hazeem NZA. Nanofibers and Electrospinning Method. IntechOpen; 2018. [CrossRef]
- Chinnappan BA, Krishnaswamy M, Xu H, Hoque ME. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers (Basel) 2022;14:3719. [CrossRef]
- Yarin A, Koombhongse S, Reneker D. Taylor Cone and Jetting from Liquid Droplets in Electrospinning of Nanofibers. Journal of Applied Physics 2001;90:4836–46. [CrossRef]
- Barhoum A, Pal K, Rahier H, Uludag H, Kim IS, Bechelany M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Applied Materials Today 2019;17:1–35. [CrossRef]
- Nayl AA, Abd-Elhamid AI, Awwad NS, Abdelgawad MA, Wu J, Mo X, et al. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers (Basel) 2022;14:1594. [CrossRef]
- Dokuchaeva AA, Timchenko TP, Karpova EV, Vladimirov SV, Soynov IA, Zhuravleva IY. Effects of Electrospinning Parameter Adjustment on the Mechanical Behavior of Poly-ε-caprolactone Vascular Scaffolds. Polymers (Basel) 2022;14:349. [CrossRef]
- Li H, Chen X, Lu W, Wang J, Xu Y, Guo Y. Application of Electrospinning in Antibacterial Field. Nanomaterials (Basel) 2021;11:1822. [CrossRef]
- Luraghi A, Peri F, Moroni L. Electrospinning for drug delivery applications: A review. J Control Release 2021;334:463–84. [CrossRef]
- Xue J, Wu T, Dai Y, Xia Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem Rev 2019;119:5298–415. [CrossRef]
- Sun G, Sun L, Xie H, Liu J. Electrospinning of Nanofibers for Energy Applications. Nanomaterials (Basel) 2016;6:129. [CrossRef]
- Zhang C, Feng F, Zhang H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends in Food Science & Technology 2018;80:175–86. [CrossRef]
- Tian Y, Zhou J, He C, He L, Li X, Sui H. The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes 2022;10:738. [CrossRef]
- Wu Y-K, Wang L, Fan J, Shou W, Zhou B-M, Liu Y. Multi-Jet Electrospinning with Auxiliary Electrode: The Influence of Solution Properties. Polymers (Basel) 2018;10:572. [CrossRef]
- Varesano A, Carletto RA, Mazzuchetti G. Experimental investigations on the multi-jet electrospinning process. Journal of Materials Processing Technology 2009;209:5178–85. [CrossRef]
- Mohammadalizadeh Z, Bahremandi-Toloue E, Karbasi S. Recent advances in modification strategies of pre- and post-electrospinning of nanofiber scaffolds in tissue engineering. Reactive and Functional Polymers 2022;172:105202. [CrossRef]
- Ding B, Kimura E, Sato T, Fujita S, Shiratori S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004;45:1895–902. [CrossRef]
- Xue J, Xie J, Liu W, Xia Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc Chem Res 2017;50:1976–87. [CrossRef]
- Li D, Yue G, Li S, Liu J, Li H, Gao Y, et al. Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers. Engineering 2022;13:116–27. [CrossRef]
- Yu D-G, Li J-J, Zhang M, Williams GR. High-quality Janus nanofibers prepared using three-fluid electrospinning. Chem Commun (Camb) 2017;53:4542–5. [CrossRef]
- Yu D-G, Yang C, Jin M, Williams GR, Zou H, Wang X, et al. Medicated Janus fibers fabricated using a Teflon-coated side-by-side spinneret. Colloids Surf B Biointerfaces 2016;138:110–6. [CrossRef]
- Zheng X, Kang S, Wang K, Yang Y, Yu D-G, Wan F, et al. Combination of structure-performance and shape-performance relationships for better biphasic release in electrospun Janus fibers. International Journal of Pharmaceutics 2021;596:120203. [CrossRef]
- Lu Y, Huang J, Yu G, Cardenas R, Wei S, Wujcik EK, et al. Coaxial electrospun fibers: applications in drug delivery and tissue engineering. WIREs Nanomed Nanobiotechnol 2016;8:654–77. [CrossRef]
- Pant B, Park M, Park S-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019;11:305. [CrossRef]
- Rathore P, Schiffman JD. Beyond the Single-Nozzle: Coaxial Electrospinning Enables Innovative Nanofiber Chemistries, Geometries, and Applications. ACS Appl Mater Interfaces 2021;13:48–66. [CrossRef]
- Liu Y, Chen X, Liu Y, Gao Y, Liu P. Electrospun Coaxial Fibers to Optimise the Release of Poorly Water-Soluble Drug. Polymers 2022;14:469. [CrossRef]
- Baykara T, Taylan G. Coaxial electrospinning of PVA/Nigella seed oil nanofibers: Processing and morphological characterisation. Materials Science and Engineering: B 2021;265:115012. [CrossRef]
- Liu W, Ni C, Chase D, Rabolt J. Preparation of Multilayer Biodegradable Nanofibers by Triaxial Electrospinning. ACS Macro Letters 2013;2:466–8. [CrossRef]
- Liu X, Yang Y, Yu D-G, Zhu M-J, Zhao M, Williams GR. Tunable zero-order drug delivery systems created by modified triaxial electrospinning. Chemical Engineering Journal 2019;356:886–94. [CrossRef]
- Yu D, Wang M, Xiaoyan L, Liu X, Zhu L-M, Bligh S. Multifluid electrospinning for the generation of complex nanostructures. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2019;12:e1601. [CrossRef]
- Zulkifli MZA, Nordin D, Shaari N, Kamarudin SK. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023;15:2418. [CrossRef]
- Zhang X, Chi C, Chen J, Zhang X, Gong M, Wang X, et al. Electrospun quad-axial nanofibers for controlled and sustained drug delivery. Materials & Design 2021;206:109732. [CrossRef]
- Miletić A, Pavlić B, Ristić I, Zeković Z, Pilić B. Encapsulation of Fatty Oils into Electrospun Nanofibers for Cosmetic Products with Antioxidant Activity. Applied Sciences 2019;9:2955. [CrossRef]
- Wilk S, Benko A. Advances in Fabricating the Electrospun Biopolymer-Based Biomaterials. J Funct Biomater 2021;12:26. [CrossRef]
- White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol 2011;29:37–42. [CrossRef]
- Clark EW. A brief history of lanolin. Pharm Hist (Lond) 1980;10:5–6.
- Ertas IF, Uzun M, Altan E, Kabir MH, Gurboga M, Ozakpinar OB, et al. Investigation of silk fibroin-lanolin blended nanofibrous structures. Materials Letters 2023;330:133263. [CrossRef]
- Thau P. Glycerin (glycerol): Current insights into the functional properties of a classic cosmetic raw material. J Cosmet Sci 2002;53:229–36.
- Fluhr JW, Gloor M, Lehmann L, Lazzerini S, Distante F, Berardesca E. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol 1999;79:418–21. [CrossRef]
- Gonçalves MM, Lobsinger KL, Carneiro J, Picheth GF, Pires C, Saul CK, et al. Morphological study of electrospun chitosan/poly(vinyl alcohol)/glycerol nanofibres for skin care applications. International Journal of Biological Macromolecules 2022;194:172–8. [CrossRef]
- Movahedi M, Asefnejad A, Rafienia M, Khorasani MT. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. International Journal of Biological Macromolecules 2020;146:627–37. [CrossRef]
- Fan L, Cai Z, Zhang K, Han F, Li J, He C, et al. Green electrospun pantothenic acid/silk fibroin composite nanofibers: fabrication, characterisation and biological activity. Colloids Surf B Biointerfaces 2014;117:14–20. [CrossRef]
- Gehring W, Gloor M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study. Arzneimittelforschung 2000;50:659–63. [CrossRef]
- Biro K, Thaçi D, Ochsendorf FR, Kaufmann R, Boehncke W-H. Efficacy of dexpanthenol in skin protection against irritation: a double-blind, placebo-controlled study. Contact Dermatitis 2003;49:80–4. [CrossRef]
- Kazsoki A, Palcsó B, Alpár A, Snoeck R, Andrei G, Zelkó R. Formulation of acyclovir (core)-dexpanthenol (sheath) nanofibrous patches for the treatment of herpes labialis. Int J Pharm 2022;611:121354. [CrossRef]
- Kligman AM. Dermatologic uses of urea. Acta Derm Venereol 1957;37:155–9.
- Krysiak Z, Stachewicz U. Urea-Based Patches with Controlled Release for Potential Atopic Dermatitis Treatment. Pharmaceutics 2022;14. [CrossRef]
- Jin YH, Lee SJ, Chung MH, Park JH, Park YI, Cho TH, et al. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch Pharm Res 1999;22:232–6. [CrossRef]
- Wahedi HM, Jeong M, Chae JK, Do SG, Yoon H, Kim SY. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine 2017;28:19–26. [CrossRef]
- Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther 2007;20:308–13. [CrossRef]
- Kumar L, Verma S, Joshi K, Utreja P, Sharma S. Nanofiber as a novel vehicle for transdermal delivery of therapeutic agents: challenges and opportunities. Future Journal of Pharmaceutical Sciences 2021;7:175. [CrossRef]
- Hazra B, Sarkar R, Biswas S, Mandal N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement Altern Med 2010;10:20. [CrossRef]
- Sancheti G, Jindal A, Kumari R, Goyal PK. Chemo-preventive action of emblica officinalis on skin carcinogenesis in mice. Asian Pac J Cancer Prev 2005;6:197–201.
- Lin J-H, Shiu B-C, Hsu P-W, Lou C-W, Lin J-H. PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations. Polymers 2022;14:4470. [CrossRef]
- Bhattarai N, Edmondson D, Veiseh O, Matsen F, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005;26:6176–84. [CrossRef]
- Zhu W, Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J Investig Dermatol Symp Proc 2008;13:20–4. [CrossRef]
- Gupta S, Dutta P, Acharya V, Prasad P, Roy A, Bit A. Accelerating skin barrier repair using novel bioactive magnesium-doped nanofibers of non-mulberry silk fibroin during wound healing. Journal of Bioactive and Compatible Polymers 2022;37:38–52. [CrossRef]
- Liu Y, Qin Y, Bai R, Zhang X, Yuan L, Liu J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. International Journal of Biological Macromolecules 2019;134:993–1001. [CrossRef]
- Shindo Y, Witt E, Han D, Epstein W, Packer L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol 1994;102:122–4. [CrossRef]
- Pullar JM, Carr AC, Vissers MCM. The Roles of Vitamin C in Skin Health. Nutrients 2017;9:866. [CrossRef]
- Fathi-Azarbayjani A, Qun L, Chan YW, Chan SY. Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. AAPS PharmSciTech 2010;11:1164–70. [CrossRef]
- Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, Miyamoto K, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol 2002;147:20–31. [CrossRef]
- Nada A, Hassabo A, Mohamed A, Zaghloul S. Encapsulation of Nicotinamide into Cellulose Based Electrospun Fibers. Journal of Applied Pharmaceutical Science 2016;6:013–21. [CrossRef]
- Katiyar SK, Ahmad N, Mukhtar H. Green tea and skin. Arch Dermatol 2000;136:989–94. [CrossRef]
- Sadri.Minoo, Arab-Sorkhi S, Vatani H, Bagheri Pebdeni A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers and Polymers 2015;16:1742–50. [CrossRef]
- Ulusoy S, Boşgelmez-Tinaz G, Seçilmiş-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr Microbiol 2009;59:554–8. [CrossRef]
- Zarshenas MM, Moein M, Zomorodian K, Almasi M, Pakshir K. Preparation and Analysis of Rosa damascena Essential Oil Composition and Antimicrobial Activity Assessment of Related Fractions. Iranian Journal of Science and Technology (Sciences) 2017;41. [CrossRef]
- Lin Y-C, Hu S, Huang P-H, Lin T-C, Yen F-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020;12:552. [CrossRef]
- Torras MAC, Faura CA, Schönlau F, Rohdewald P. Antimicrobial activity of Pycnogenol. Phytother Res 2005;19:647–8. [CrossRef]
- Wang A, Pope SD, Weinstein JS, Yu S, Zhang C, Booth CJ, et al. Specific sequences of infectious challenge lead to secondary hemophagocytic lymphohistiocytosis-like disease in mice. Proc Natl Acad Sci U S A 2019;116:2200–9. [CrossRef]
- Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol 2008;7:2–7. [CrossRef]
- De Spirt S, Stahl W, Tronnier H, Sies H, Bejot M, Maurette J-M, et al. Intervention with flaxseed and borage oil supplements modulates skin condition in women. Br J Nutr 2009;101:440–5. [CrossRef]
- Hadad S, Goli S. Improving Oxidative Stability of Flaxseed Oil by Encapsulation in Electrospun Flaxseed Mucilage Nanofiber. Food and Bioprocess Technology 2019;12. [CrossRef]
- Hadad S, Goli S. Fabrication and characterisation of electrospun nanofibers using flaxseed ( Linum usitatissimum ) mucilage. International Journal of Biological Macromolecules 2018;114. [CrossRef]
- Staniforth V, Chiu L-T, Yang N-S. Caffeic acid suppresses UVB radiation-induced expression of interleukin-10 and activation of mitogen-activated protein kinases in mouse. Carcinogenesis 2006;27:1803–11. [CrossRef]
- Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, et al. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm 2000;199:39–47. [CrossRef]
- Vilchez A, Acevedo F, Cea M, Seeger M, Navia R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020;10:175. [CrossRef]
- Kaya S, Yilmaz DE, Akmayan I, Egri O, Arasoglu T, Derman S. Caffeic Acid Phenethyl Ester Loaded Electrospun Nanofibers for Wound Dressing Application. J Pharm Sci 2022;111:734–42. [CrossRef]
- Wang Q-J, Gao X, Gong H, Lin X-R, Saint-Leger D, Senee J. Chemical stability and degradation mechanisms of ferulic acid (F.A) within various cosmetic formulations. J Cosmet Sci 2011;62:483–503.
- Vashisth P, Kumar N, Sharma M, Pruthi V. Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol Rep (Amst) 2015;8:36–44. [CrossRef]
- Charurin P, Ames JM, del Castillo MD. Antioxidant activity of coffee model systems. J Agric Food Chem 2002;50:3751–6. [CrossRef]
- Sheng X, Fan L, He C, Zhang K, Mo X, Wang H. Vitamin E-loaded silk fibroin nanofibrous mats fabricated by green process for skin care application. Int J Biol Macromol 2013;56:49–56. [CrossRef]
- Taepaiboon P, Rungsardthong U, Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur J Pharm Biopharm 2007;67:387–97. [CrossRef]
- Bhagavathula N, Warner RL, DaSilva M, McClintock SD, Barron A, Aslam MN, et al. A combination of curcumin and ginger extract improves abrasion wound healing in corticosteroid-impaired hairless rat skin. Wound Repair Regen 2009;17:360–6. [CrossRef]
- Jacob J, Haponiuk J, Thomas S, Peter G, Gopi S. Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers 2018;6:79. [CrossRef]
- Jacob J, Peter G, Thomas S, Haponiuk JT, Gopi S. Chitosan and polyvinyl alcohol nanocomposites with cellulose nanofibers from ginger rhizomes and its antimicrobial activities. Int J Biol Macromol 2019;129:370–6. [CrossRef]
- Israili ZH. Antimicrobial properties of honey. Am J Ther 2014;21:304–23. [CrossRef]
- Ediriweera ERHSS, Premarathna NYS. Medicinal and cosmetic uses of Bee’s Honey - A review. Ayu 2012;33:178–82. [CrossRef]
- Pakolpakçıl A, Draczynski Z. Green Approach to Develop Bee Pollen-Loaded Alginate Based Nanofibrous Mat. Materials 2021;14:2775. [CrossRef]
- Ionescu OM, Mignon A, Iacob AT, Simionescu N, Confederat LG, Tuchilus C, et al. New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterisation and In Vitro Biological Evaluation. Polymers (Basel) 2021;13:1291. [CrossRef]
- Pugazhenthi K, Kapoor M, Clarkson AN, Hall I, Appleton I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J Pineal Res 2008;44:387–96. [CrossRef]
- Morganti P. Melatonin and immunostimulating substance-based compositions. EP1991222B1, 2016.
- Mirmajidi T, Chogan F, Rezayan AH, Sharifi AM. In vitro and in vivo evaluation of a nanofiber wound dressing loaded with melatonin. Int J Pharm 2021;596:120213. [CrossRef]
- Rahman S, Carter P, Bhattarai N. Aloe Vera for Tissue Engineering Applications. JFB 2017;8:6. [CrossRef]
- Barbosa R, Villarreal A, Rodriguez C, De Leon H, Gilkerson R, Lozano K. Aloe Vera extract-based composite nanofibers for wound dressing applications. Mater Sci Eng C Mater Biol Appl 2021;124:112061. [CrossRef]
- Cavanagh HMA, Wilkinson JM. Biological activities of lavender essential oil. Phytother Res 2002;16:301–8. [CrossRef]
- Wells R, Truong F, Adal AM, Sarker LS, Mahmoud SS. Lavandula Essential Oils: A Current Review of Applications in Medicinal, Food, and Cosmetic Industries of Lavender. Natural Product Communications 2018;13:1934578X1801301038. [CrossRef]
- Sofi HS, Akram T, Tamboli AH, Majeed A, Shabir N, Sheikh FA. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int J Pharm 2019;569:118590. [CrossRef]
- Uhlířová R, Langová D, Bendová A, Gross M, Skoumalová P, Márová I. Antimicrobial Activity of Gelatin Nanofibers Enriched by Essential Oils against Cutibacterium acnes and Staphylococcus epidermidis. Nanomaterials 2023;13:844. [CrossRef]
- Liu H, Yu H, Xia J, Liu L, Liu GJ, Sang H, et al. Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc and fruit acid (alpha-hydroxy acid) for acne. Cochrane Database Syst Rev 2020;5:CD011368. [CrossRef]
- Yaru W, Lan X, Jianhua S, Chenxu F. Preparation, Characterization and Drug Release of Salicylic Acid Loaded Porous Electrospun Nanofibers. Recent Patents on Nanotechnology 2018;12. [CrossRef]
- Gonçalves S. Use of enzymes in cosmetics: proposed enzymatic peel procedure 2021;1:29–35.
- Bié J, Sepodes B, Fernandes PCB, Ribeiro MHL. Enzyme Immobilisation and Co-Immobilization: Main Framework, Advances and Some Applications. Processes 2022;10:494. [CrossRef]
- Basso A, Serban S. Overview of Immobilized Enzymes’ Applications in Pharmaceutical, Chemical, and Food Industry. Methods Mol Biol 2020;2100:27–63. [CrossRef]
Figure 7.
Encapsulated nanofibers with active ingredients.
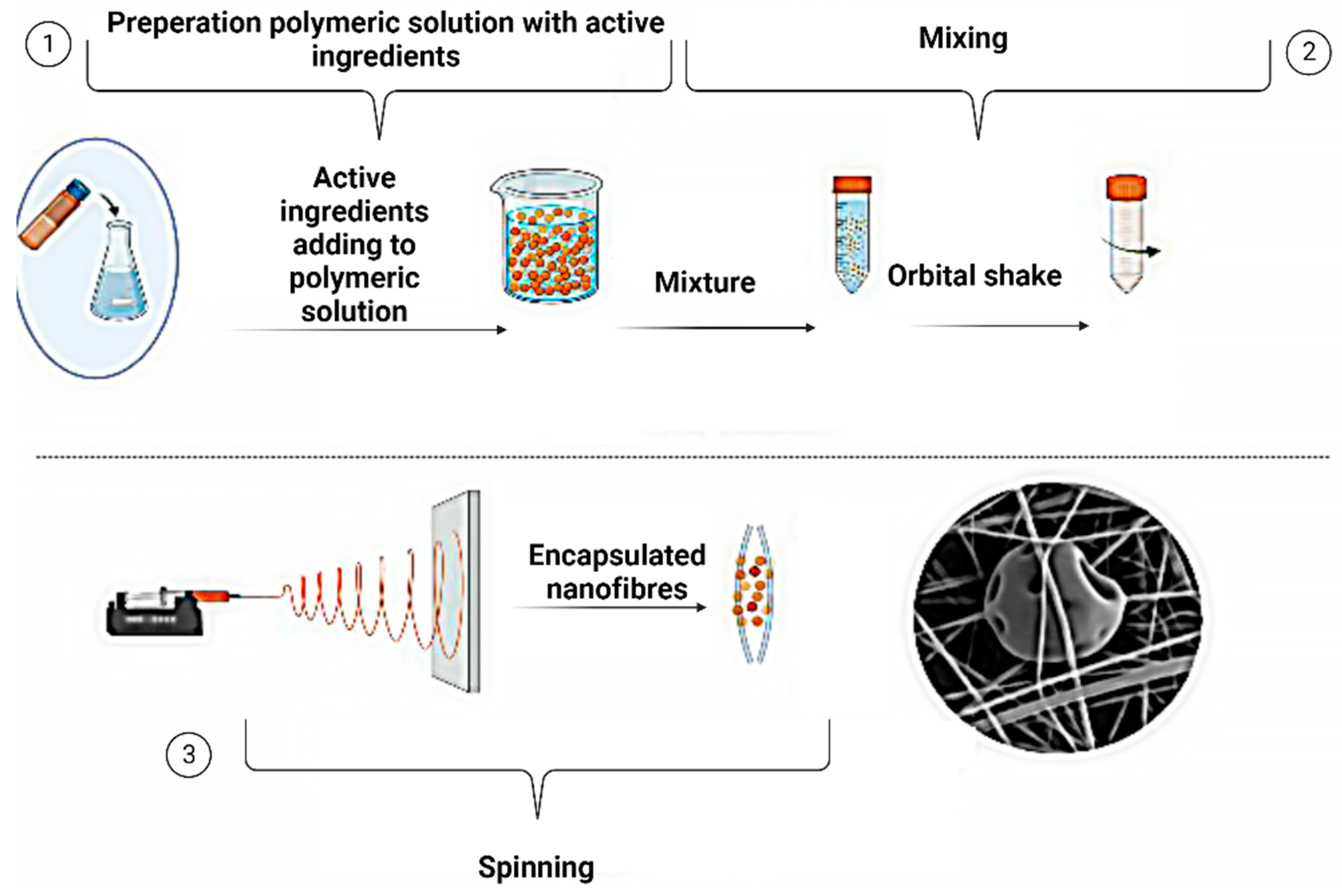
Figure 8.
Schematic representation of various methods for incorporating drugs into a polymer carrier using electrospinning.
Figure 8.
Schematic representation of various methods for incorporating drugs into a polymer carrier using electrospinning.

Figure 9.
Cross-sections of electrospun fibres form for active ingredients encapsulation.
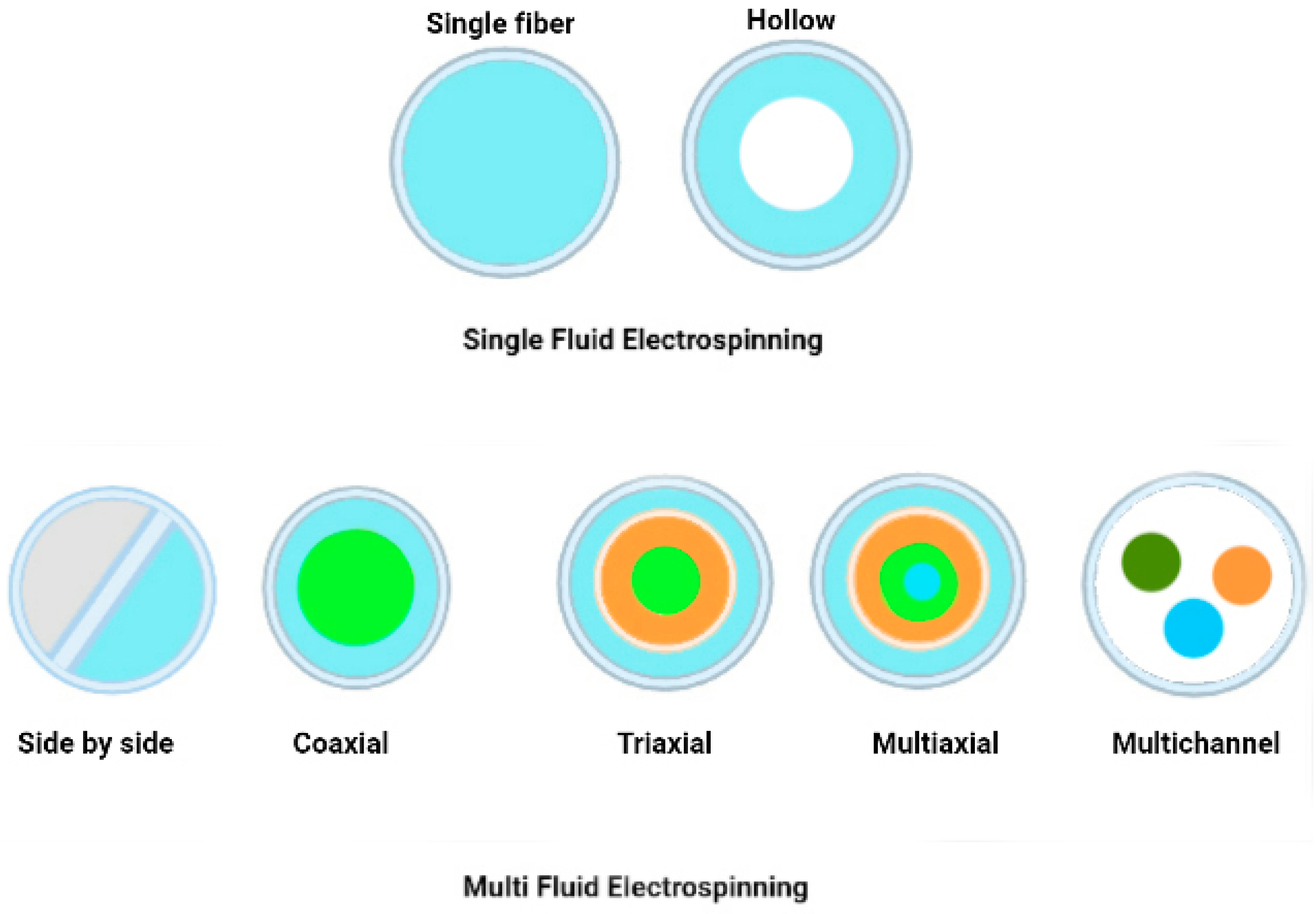
Table 1.
Active Ingredients from Natural Sources for Different Skin Types.
| Skin Type | Active Ingredient | Benefits | References |
|---|---|---|---|
| Dry skin | Aloe vera | Moisturises and soothes dry skin and helps restore the natural skin moisture barrier. | [30] |
| Chamomile | It has anti-inflammatory properties and soothes dry, irritated skin. | [31] | |
| Calendula | Helps to hydrate and heal dry, damaged skin. | [32] | |
| Lavender | Has calming properties and soothes dry, itchy skin. | [33] | |
| Coconut oil | Has moisturising properties and helps to soothe and hydrate dry skin. | [34] | |
| Jojoba oil | Helps to moisturise dry skin without leaving a greasy residue | [35] | |
| Shea butter | Has deeply moisturising properties, helps to soothe and nourish dry skin | [36] | |
| Olive oil | Contains antioxidants and moisturising properties, helps to hydrate and protect dry skin | [37] | |
| Rosehip oil | Contains essential fatty acids and vitamin A, helps to hydrate and rejuvenate dry skin | [38] | |
| Witch hazel | Has astringent properties, helps to tighten and tone dry, ageing skin | [39] | |
| Normal skin | Niacinamide | Helps improve skin texture and tone, reduces the appearance of fine lines and wrinkles, and strengthens the skin barrier | [40] |
| Vitamin C | Helps brighten and even out skin tone, promotes collagen synthesis, and protects against environmental damage | [41] | |
| Hyaluronic acid | Provides deep hydration and helps retain moisture in the skin, improving skin elasticity and firmness. | [42] | |
| Green tea extract | Has anti-inflammatory and antioxidant properties, helps protect against UV damage, and promotes healthy skin ageing | [43] | |
| Retinoids | Helps stimulate collagen production, improves skin texture and tone, and reduces the appearance of fine lines and wrinkles | [44] | |
| Oily skin | Salicylic acid | Helps unclog pores, reduces oiliness, and prevents breakouts | [45] |
| Tea tree oil | Has anti-inflammatory and antimicrobial properties, helps reduce acne and oiliness | [46] | |
| Zinc | Helps regulate sebum production, has anti-inflammatory and antimicrobial properties, and promotes wound healing. | [47] | |
| Witch hazel | Has astringent properties that can help tighten and tone oily skin, reduces inflammation and irritation | [39] | |
| Combination | Niacinamide | Helps regulate sebum production, improves skin texture and tone, and strengthens the skin barrier. | [40] |
| Hyaluronic acid | Provides deep hydration to dry areas while being lightweight enough not to exacerbate oiliness in the T-zone | [42] | |
| Vitamin C | Helps brighten and even out skin tone, promotes collagen synthesis, and protects against environmental damage | [41] | |
| Alpha-hydroxy acids (AHAs) | Help exfoliate dead skin cells, improve skin texture and tone, and reduce the appearance of fine lines and wrinkles. | [40] | |
| Jojoba oil | A similar structure to the natural skin sebum helps regulate oil production and hydrates dry areas. | [35] |
Table 2.
The sum of active ingredients and their benefits to the skin.
| Electrospun polymers | Ingredients | Benefits for skin | Personal Care Category | References |
|---|---|---|---|---|
| Silk fibroin | Lanolin | Occlusive, emollient | Lipophilic | [150,151,152] |
| Chitosan, PVA | Glycerine | Anti-inflammatory, barrier recovery | Humectant, moisturiser | [153,154,155] |
| Chitosan, Gelatin, and PVA | Hyaluronic Acid | Humectant, anti-aging | Humectant, moisturiser | [42,156] |
| Silk fibroin | Vitamin B5 (Pantothenic Acid/Dexpanthenol) | Hydration, barrier protection, reduction of trans-epidermal water loss (TEWL), fibroblast stimulation, and re-epithelialisation. | Humectant, emollient, antiinflammatory | [157,158,159,160] |
| PVA, Gelatin | Urea | Anti-inflammatory, hydrating, keratolytic | Humectant, emollient | [161,162] |
| Chitosan,Gelatin, and PVA | Aloesin | Tyrosinase inhibition, antioxidant, anti-inflammatory | Depigmenting, sun protective (UVB) | [163,164] |
| PVA | Hydroquinone | Tyrosinase inhibition | Depigmenting, brightening, lightening | [165,166] |
| Chitosan | Emblica Extract | Antioxidant, anti-inflammatory, antipyretic, antitumor, chemo-preventive, hepatoprotective, analgesic, antibacterial | Depigmenting, anti-ageing, sunscreen | [167,168,169,170] |
| PVA, PCL, Chitosan | Mulberry Extract | Antityrosinase, antihyperglycemic, antitumorigenic, anti-inflammatory, antipyretic, antioxidant, anti-atherogenic, antimicrobial chemo-preventive, neuro-protective | Depigmenting | [171,172,173] |
| PVA | Vitamin C (Ascorbic Acid) | Anti-inflammatory, antioxidant, photo-protectant, depigmenting | Depigmenting | [174,175,176] |
| PVA | Niacinamide | PAR-2 inhibition, anti-inflammatory, antioxidant, anti-ageing, photoprotective | Depigmenting, exfoliant | [171,177,178] |
| PVA, Chitosan | Green Tea | Antioxidant, anti-ageing, antiacne, antiangiogenic, anticarcinogenic, anticarcinogenic, anti-inflammatory, antimicrobial, chemo-preventive, immunomodulatory,2photoprotective | Anti-ageing, moisturising, antiacne, anogenital wart treatment | [43,179,180] |
| PVA.Chitosan | Rosa Damascena | Antioxidant, antibacterial, antimicrobial, anti-inflammatory, antiseptic, and anxiolytic | Antioxidant | [181,182] |
| PVA, PLGA | Pycnogenol | Antioxidant, anti-inflammatory, anticarcinogenic, photoprotective, antimicrobial | Anti-inflammatory, hydrating | [183,184,185] |
| PVA, PLGA | Resveratrol | Antioxidant, antibacterial, anticancer, antiinflammatory, antitumorigenic, anti-tyrosinase, cardioprotective | Anti-ageing, anticancer | [186] |
| PVA, PLGA.Chitosan | Flaxseed Oil | Antioxidant, anti-ageing, anti-inflammatory, and antiapoptotic | Antioxidant, anti-ageing | [187,188,189] |
| PVA, PLGA | Caffeic Acid | Antioxidant, anticarcinogenic, anti-inflammatory, antimicrobial, immunostimulatory, neuroprotective, photoprotective | Antioxidant, anti-ageing | [190,191,192,193] |
| PLGA.PEO | Ferulic Acid | Antioxidant, anticancer, anti-inflammatory, antimicrobial, cardioprotective, neuroprotective, hepatoprotective, photoprotective, skin lightening | Antioxidant, anti-ageing, photoprotection | [192,194,195] |
| Cellulose acetate | Tocopherol (Vitamin E) | Antioxidant, photoprotection, wound healing | Antioxidant, moisturising, anti-ageing | [196,197,198] |
| Chitosan, PVA | Ginger | Antioxidant, anticarcinogenic, anti-inflammatory, antinausea, wound healing | Antioxidant, analgesic, photo-protectant | [199,200,201] |
| Hyaluronic acid, PEO | Honey/Propolis/Royal Jelly | Analgesic, antioxidant, antiinflammatory, antimicrobial, antitumor, antiseptic, antipyretic, antiulcer, hepatoprotective, immunomodulatory |
Antioxidant, anti-ageing, photoprotection, antiseptic, wound healing | [202,203,204,205] |
| Chitosan, Polycaprolactone,PVA | Melatonin | Antioxidant, anticarcinogenic, anti-ageing, anti-inflammatory, anxiolytic, immunomodulatory | Antioxidant, anti-ageing | [206,207,208] |
| Pullulan | Aloe Vera | Anti-inflammatory, antioxidant, antimicrobial, immunomodulatory, laxative, wound healing | Moisturising, soothing, cooling, burning and wound healing | [30,209,210] |
| Polyurethane, Gelatin | Lavandula | Anti-inflammatory, antimicrobial, antiseptic, anti-colic, antispasmodic, antidepressant, sedative | Soothing, sedating, anti-inflammatory, analgesic | [211,212,213,214] |
| PLA | Salicylic Acid | Anti-inflammatory, pore cleansing | Cleansing, antiacne, pore minimising, exfoliating | [215,216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Exploring the Efficacy of AHA-BHA Infused Nanofiber Skin Masks as a Topical Treatment for Acne Vulgaris
Elçin Tören
et al.
,
2023
Development of Topical Formulations Containing 20% of Coated and Uncoated Zinc Oxide Nanoparticles: Stability Assessment and Penetration Evaluation by Reflectance Confocal Laser Microscopy
Camila Helena Ferreira Cuelho
et al.
,
2023
Elevating Skincare Science: Grape Seed-Extract Encapsulation for Dermatological Care
Maria Leonor de Castro
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









