Preprint
Article
Bioaccumulation of Microcystin-LR and Induced Physio-Biochemical Changes in Rice (Oryza sativa L.) at Vegetative Stage under Hydroponic Culture Conditions
Altmetrics
Downloads
92
Views
31
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Irrigation with water containing a variety of microcystins (MCs) may pose potential threat to normal growth of agricultural plants. The mechanism of microcystin-LR (MC-LR) induced phytotoxicity in rice (Oryza sativa L.) at environmental concentrations is still unknown. Rice seedlings were exposed to MC-LR at concentrations of 0.10, 1.0, 10.0 and 50.0 μg·L−1 in hydroponic nutrient solutions for 7, 15, 20, and 34 days in the current study. The absorption and accumulation in leaf and root tissues, as well as a series of key physio-biochemical process changes in leaves of rice at different exposure time points were measured. Results showed that MC-LR could be detected in rice leaves and roots in all exposure groups, however, a significant accumulation trend of MC-LR in plants (BCF > 1) was only found in the lowest group (0.10 μg·L−1). The time-course study revealed a biphasic response of O2•- levels in rice leaves to the exposure of MC-LR, which was more pronounced in higher concentration groups, which could be attributed to the combined effects of antioxidant and detoxification mechanisms in rice. Exposure to 1.0 - 50.0 μg·L−1 MC-LR resulted in significant depletion of GSH and MDA contents in rice leaves at later exposure times. The changes of nitric oxide synthase (NOS) in rice leaves under MC-LR exposure were firstly investigated in the current study. Low MC-LR concentrations promoted NOS activity, whereas high concentrations inhibited NOS activity during the later exposure times, implying that NO may play a role in MC-LR toxicity in rice. Reduced sucrose synthase (SS) activities in rice exposed to MC-LR can reduce the plant's ability to accumulate carbon and thus may be directly related to the reduction in vegetative growth. These findings suggest that even at low concentrations of MC-LR, terrestrial plants' normal physiological status is disrupted, which, when combined with previous findings, helps reveal the mechanism of MC-LR-induced phytotoxicity.
Keywords:
Subject: Environmental and Earth Sciences - Environmental Science
1. Introduction
In recent years, numerous evidence has shown that the harmful cyanobacterial blooms are rising in frequency, magnitude and duration across the world [1,2,3]. Cyanobacterial blooms have influenced worldwide freshwater ecosystems during the last few decades. Common taxa in the bloom-forming cyanobacteria species (BFCS) include species of Microcystis, Anabaena, Nodularia, and Cylindrospermopsis, etc. [3,4,5]. As the most common BFCS, Microcystis producing toxic secondary metabolites as microcystins (MCs), has received the most attention and research. MCs are potent hepatotoxins with over 90 different variants [1,6], the most toxic and prevalent of which are microcystin-LR (MC-LR), MC-RR, and MC-YR [7,8]. During bloom outbreaks, dissolved MCs concentrations in water typically range between 0.1 and 10 μg·L−1, while ecological risks of the low MCs concentrations in natural environments remain unclear, and the risks that associated with MCs in the food chain have not been fully elucidated [1,2,9]. Moreover, MCs can enter farmland via irrigation water and will inevitably harm terrestrial plants [10,11], however, very little known about the mechanism of MC-induced phytotoxicity on terrestrial plants [12,13,14,15].
Current research on the toxic effects and mechanisms of MCs toxicity is primarily concerned with their effects on animals and human health. MCs fate in natural environments can lead to its transfer into aquatic or terrestrial organisms [9,16]. Previous research has already shown that MCs can be transferred into terrestrial plants by farmland irrigation, e.g., spray irrigation, and thus may accumulate the toxins in the plant body [17]. MC contents in crop products (Capsicum annuum and Solanum lycopersicum) irrigated with water contaminated by cyanobacterial blooms were found to be as high as 8.10 ± 4.67 μg·kg−1 DW (dry weight) [18]. Chen found that MC-LR concentrations in rice seed samples collected in the Lake Taihu area ranged from 0.04 to 3.19 μg·kg−1 [13]. Uptake and accumulation of cyanobacterial toxins in plants will inevitably trigger a series of physiological and biochemical reactions that can inhibit plant growth, causing the reduction of the quality and productivity of terrestrial agricultural plants [19].
A widely acceptable mechanism MCs specifically inhibits protein phosphatases 1 and 2A in plant and animal cells, which leads to a series of toxic downstream effects [20,21,22]. The toxicity mechanism mediated by oxidative stress in plants has also been well characterized in recent years [1,9,15,23]. Exposure to 0.50–10.0 μg·L−1 of MCs in the form of cyanobacteria crude extract or pure toxin could induce the responses of antioxidant systems of plants, as evidenced by significant changes in the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione peroxidase (GPX), and glutathione S-transferase (GST) [24]. Jiang et al. [1] also demonstrated that GSH was involved in the detoxification of MC-LR in Vallisneria natans, and oxidative damage induced in plant was demonstrated by a significant increase in the malondialdehyde (MDA) content at 1.0 μg·L−1 of dissolved MC-LR. It should be emphasized that previous studies on MCs exposure have often employed far greater concentrations that what plants really face in their environment. Nitric oxide (NO) interacts in different ways with ROS (reactive oxygen species) and may play a role as an antioxidant under certain stress conditions [25,26,27]. NO is mainly synthesized and released by nitric oxide synthase (NOS) and nitrate reductase, among which iNOS plays a dominant transcriptional role and synthesizes most of the cellular NO [28]. So far the relationship between MC-LR exposure and NO generation has not been reported in plants.
Current research on the impact of environmental concentrations of MC-LR accumulation in terrestrial plants is not extensive, nor is the research on the MCs induced physiological and biochemical responses in plants. Previous research has demonstrated that low concentrations of MC-LR promote the increase of rice biomass (including plant height, root length, and fresh weight), while at higher concentrations, MC-LR plays an inhibitory role on vegetative growth, leaving the plants small, with yellow leaves [10]. The current work aims delve further into the characteristics of absorption and accumulation in leaf and root tissues, as well as a series of key physio-biochemical process changes in leaves of rice seedlings under long-term exposure of MC-LR.
2. Materials and Methods
2.1. Chemicals and reagents
Microcystin-LR, ≥ 95% in purity (HPLC) was purchased from Taiwan algae Institute Ltd. (Taiwan Algal Science Inc). Microcystin Plate Kit was purchased from Institute of Hydrobiology, Chinese Academy of Sciences. Phenylmethylsulfonyl fluoride (PMSF), o-phthaldialdehyde, bovine serum albumin (BSA), polyvinylpolypyrrolidone (PVPP), and α-naphthylamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, acetic acid, and dimethyl sulfoxide (DMSF) was purchased from Merck (Darmstadt, Germany). Other reagents are of analytical grade and were purchased from Chinese companies. All solutions were formulated with Milli-Q water.
2.2. Experimental rice and MC-LR exposure
The Nipponbare rice variety (Oryza sativa L.) was used for the following experiments. One thousand robust and full Nipponbare rice seeds were selected and divided into four groups. The seeds were soaked at 28ºC for 24 hours and germination was induced by covering the rice seeds with three layers of wet gauze and placing them into containers lined with aluminum foil, so that the seeds would be in a dark and humid environment. The container was placed in a 25ºC incubator to induce germination and the seeds were kept in a humid environment throughout the germination process. Rice seedlings were transferred to the International Rice Research Institute in conventional nutrient solution after germination (containing 40 mg·L−1 Na+, 40 mg·L−1 K+, 40 mg·L−1 Ca2+, 40 mg·L−1 Mg2+, 10 mg·L−1 P5+, 2 mg·L−1 Fe3+, 0.5 mg·L−1 Mn2+, 0.05 mg·L−1 Mo6+, 0.2 mg·L−1 B3+, 0.01 mg·L−1 Zn2+, and 0.01 mg·L−1 Cu2+) and cultured.
Plant seedlings were cultured in nutrient solution until 5 days after germination. Plants that were growing well were selected, cleaned with ultrapure water, and subjected to the MC-LR exposure test. The test groups were exposed to MC-LR concentrations of 0.10, 1.00, 10.0, and 50.0 μg·L−1, while a negative control group was exposed to nutrient solution without MC-LR. All seedlings were exposed for 7, 15, 20, or 34 days and four parallel subgroups were defined in each group. During the exposure period, the nutrient solution containing MC-LR was replaced every other day. The initial solution was 100 ml of diluted nutrient solution, but with plant growth, the dilution was gradually reduced and the nutrient solution was increased to 200 mL to ensure that the plants had sufficient nutrients for growth. At the end of the exposure period, the plant biomass was measured immediately. Fresh leaves and roots were taken, quick-frozen under liquid nitrogen, packaged, and kept in a -80ºC freezer.
2.3. Microcystin analysis
The accumulated MC-LR in plant samples was analyzed by ELISA method with Microcystin Plate Kit according to Jiang et al. [1].
2.4. Determination of superoxide anion (O2•-) Content
On days 10, 20, and 34 after exposure to MC-LR, about 0.2 g fresh leaves were collected and accurately weighed, then quickly pulverized to powder after under liquid nitrogen. A five-fold volume of 50 mmol·L−1 phosphate buffer (pH 7.8) containing 0.1 mmol·L−1 EDTA, 4% (W/V) PVPP, and 0.3% (W/V) Triton X-100 was added to make a homogenate, which was centrifuged at 10 500×g for 20 min. Immediately, 0.5 mL of the supernatant was drawn and added to 0.5 mL of phosphate buffer (50 mmol·L−1, pH 7.8) and 1 mL of 1.0 mmol·L−1 hydroxylamine hydrochloride. The mixture was shook to mix before being incubated at 25ºC for 1 hour. One milliliter of 17.0 mmol·L−1 amino acid (prepared with glacial acetic acid and water in a 3:1 ratio) and 1 mL of 7.0 mmol/L α-naphthylamine (prepared with glacial acetic acid and water in a 3:1 ratio) were added. After mixing, the sample was incubated at 25ºC for 20 minutes before its absorbance at 530 nm was measured. Using a solution of sodium nitrite, a standard curve was generated as described above, and the O2•- content was calculated based on the standard curve.
2.5. Glutathione and malondialdehyde determination
The amounts of GSH in plant leaves were measured according to Hissin and Hilf [29] with modifications according to Jiang et al. [1]. The amounts of MDA in plant leaves were determined using a biochemical assay kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
2.6. Enzyme extraction and activity assays
About 0.2 g fresh leaves were collected and quickly pulverized to a fine powder under liquid nitrogen. The extraction of crude enzyme from the powder was according to Jiang et al. [1]. The extracts was separated into aliquots and kept at 80°C for additional investigation. All operations were carried out at 0–4oC. The determination of sucrose synthase (SS, EC 2.4.1.13), inducible nitric oxide synthase (iNOS), and total nitric oxide synthase (TNOS, EC 1.14.13.39) activities was using a commercialized biochemical assay kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. The Bradford method [30] was used in every instance to determine the total protein (Pr) level using bovine serum albumin as the reference.
2.7. Data analysis
Data were checked for normality using the Shapiro-Wilk test, and when necessary, they were converted to adhere to the normal distribution assumption. With the use of SPSS 15.0 software, a one-way ANOVA test and Duncan’s multiple range test were employed to examine the statistical significance. Differences of p < 0.05 were considered significant, while p < 0.01 were considered high significant. Quadruplicate analyses of each sample were conducted. The Pearson Correlation Coefficient between the two variables was calculated using the Correlation Plot tool in Origin 2021 and the correlation heat map was drawn.
3. Results and discussion
3.1. Levels of MC-LR in rice leaves and roots
Using enzyme-linked immunosorbent assay (ELISA), accumulated MC-LR was measured in rice leaves. For the 7-day experimental group and the 50.0 μg·L−1 MC-LR group for 15 days, we did not collect enough leaves to determine the MC-LR content. MC-LR was detected in leaf and root tissues of all exposed plants groups, as shown in Table 1. By day 20, the level of MC-LR in leaf tissues increased with increasing environmental concentration. At a given exposure concentration of MC-LR, there is an initial increase in the MC-LR detected in rice leaves and then followed by a decrease in general. The amount of MC-LR in rice roots was positively correlated with the concentration of MC-LR in the nutrient solution after exposure for 7 days (r = 0.917). We did not see a linear relationship between the exposure concentration and the amount of MC-LR in rice roots at subsequent time periods. The amount of accumulated MC-LR in rice roots decreased with time in the 10.0 μg·L−1 MC-LR group. After 15 days of treatment, the group subjected to 50.0 μg·L−1 MC-LR had considerably reduced MC-LR levels in leaves and roots as compared to exposure to lower concentrations of MC-LR.
This study calculated the relative accumulation of MC-LR in the rice roots and leaves. According to Table 1, the ratio of MC-LR in plant tissues (leaves or roots) to water in the lowest concentration treatment groups (0.1 μg·L−1) were both greater than 1, suggesting that the rice would uptake and accumulate the MC-LR in leaves and roots. However, the ratios in the treatment groups with higher concentration (10.0, 50.0 mg·kg−1) were much lower than 1, indicating that the living rice did not actively uptake a significant amount of MC-LR for self-defense.
In ecosystems, MC shows bioaccumulation in zooplankton, molluscs and crustaceans, fish, and other common invertebrates and vertebrates. Animal cells have special membrane transport proteins that play a part in toxin accumulation [31], but a transporter protein has not been confirmed for MC-LR in terrestrial plants. Recent research has explored how OsOATPM aids in the exocytosis of MC-LR in plants [19,32]. Several reports on the bioaccumulation of MCs in aquatic plants (Lemna gibba and Vallisneria natans) suggests that terrestrial plants, including food crops, can also accumulate MC through irrigation water [1,33]. By irrigating with eutrophic water containing cyanobacterial blooms and dissolved toxins, terrestrial plants may become exposed to cyanobacterial toxins. Chen et al. [12] exposed rice to 24 μg·L−1 MC-LR for 10 days and did not detect any MC in rice leaves, while the same concentration resulted in higher bioaccumulation in exposed rapeseed plant leaves. Maejima et al. [34] found that broccoli plants exposed to 0.01–10 μg·mL−1 MC-RR for 7 days did not accumulate MC-RR if they were exposed to concentrations lower than 0.1 μg·mL−1. The bioaccumulation in broccoli exposed to 1, 5, and 10 μg·mL−1 of MC-RR was 14.5, 88.9, and 145 ng·mL−1, respectively. Moreover, MC-LR was discovered for the first time to be bioaccumulated in rice grain in the Taihu Lake region, where environmental MC-LR concentrations were low and did not pose a threat to drinking water safety [13]. According to our study, the bioaccumulation of MC-LR in rice leaves and roots does not follow a linear relationship with environmental MC-LR concentrations or time of exposure. Our results show that after the same period, the MC-LR in plants tissues increases with environmental concentration, but there is a sudden decrease in MC-LR in the high concentration exposure group (50.0 μg·L−1). The BCF values of toxin in leaves and roots indicated that the MC-LR would bioaccumulate in the plant tissues and roots only happened in the lowest concentration treatment groups (0.10 μg·L−1). The MC-LR bioaccumulation, exposure concentration, exposure time, and plant type are all interrelated. When the plant exposed to a higher concentrations of MC-LR, its defense mechanisms are activated. The bioaccumulated MC-LR in rice might be partially bio-transformed through a glutathione-related pathway, which was also the important detoxification pathway in aquatic plants [4,23]. The cell structure damages in plant cells caused by high concentrations of MC-LR exposure may also lead to decreased MC-LR accumulation [19,35].
3.2. Effects of MC-LR exposure on O2•- levels in rice leaves
ROS are often associated with environmental stress-related plant toxicity. Figure 1 depicts the levels of O2•- in rice leaves following MC-LR exposure. During early exposure times, the rice plants are still in the seedling stage and provide inadequate sample sizes. As a result, we began measuring the levels of O2•- after 10 days of MC-LR exposure, which was also more reflective of changes in enzymatic activities. As shown in Figure 1, after 10 days of MC-LR exposure, all groups had significantly lower levels of O2•- than the control group (p < 0.05, p < 0.01). The levels of O2•- in the treatment groups, on the other hand, were significantly higher than those in the control group (p < 0.05, p < 0.01) after 20 days of exposure, with increases of 60.7%, 27.7%, 114%, and 90.1%, respectively. By day 34, overall O2•- levels appear to be decreasing, with O2•- levels in the 1.0 and 10.0 μg·L−1 MC-LR even significantly lower when compared to the control group (p < 0.05).
ROS are produced in vivo under normal physiological conditions, reacting chemically with biological macromolecules, including unsaturated fatty acids, proteins, and DNA [1,2,35]. These reactions result in damage to macromolecules, which affect their normal physiological functions and induce oxidative stress. Under normal physiological conditions, there is a dynamic equilibrium between the ROS production and the antioxidant defense system of the organism. MCs have been shown in studies to directly induce the excessive production of ROS in aquatic plant tissues, and the ROS levels in a plant are closely related to environmental stress-induced toxicity [1,23]. The results of our study demonstrated that MC-LR indeed directly induces excessive O2•- production in rice leaves. As shown in Figure 1, after 10 days of MC-LR exposure, the concentration of O2•- decreased with increasing MC-LR concentrations, reaching a minimum when exposed to 50.0 μg·L−1 MC-LR. This is likely due to the synthesis results of the early responses of antioxidant system and damages of important subcellular structures caused by MC-LR exposure, e.g., cell membrane of mesophyll cells [1]. After 20 days of exposure, plants exposed to 0.10,10.0, and 50.0 μg·L−1 MC-LR had significantly higher O2•- levels in their leaves, indicating that the metabolism reaction and detoxification conversion of MC-LR occurred, leading to the formation of excessive ROS. Similarly, Chen et al. [13] found that 2 mg·L−1 MC-LR exposure for 2 days results in significantly higher levels of ROS in the rice root system compared to the control.
3.3. Effects of MC-LR exposure on GSH levels in rice leaves
Figure 2 depicts the changes of GSH content in rice leaves following MC-LR treatment. The GSH content in rice leaves was significantly greater after 7 days of exposure to 50.0 μg·L−1 MC-LR compared to the control (p < 0.05). On the contrary, exposure to 50.0 μg·L−1 MC-LR for 15, 20, and 34 days resulted in significantly reduced GSH levels in rice leaves as compared to the control (p < 0.05, p < 0.01). Furthermore, exposure to 0.10 μg·L−1 MC-LR for 34 days significantly increased the GSH content in rice leaves, whereas exposure to higher MC-LR concentrations of 1.0 and 10.0 μg·L−1 resulted in a significant reduction in GSH.
Numerous studies have demonstrated through in vitro cell-free systems or in vivo that, as the most abundant cellular thiol involved in the removal of ROS, GSH also plays an important role in the detoxification of MCs [1,36]. In the present study, there was a significant decrease in GSH content in the leaves of rice plants exposed to high concentrations of MC-LR after 15, 20, and 34 days of treatment (50.0 μg·L−1), indicating that GSH is involved in MC-LR detoxification, most likely by binding to MC-LR, increasing its solubility, and aiding in its removal from the organism. It is also possible that a higher concentration of MC-LR inhibits the synthesis of GSH. In addition, oxidative stress causes GSH to convert to the oxidized GSSG form, resulting in a decrease of GSH. Therefore, there are many possible reasons behind the changes in GSH content. As shown in Figure 2, after undergoing MC-LR stress for 34 days, the toxin at low concentration (0.10 μg·L−1) can also stimulate the synthesis of GSH. Thus, changes in GSH content are the result of a comprehensive systemic response.
3.4. Effects of MC-LR exposure on MDA levels in rice leaves
As a marker for lipid peroxidation, the intracellular MDA content changes when the plant is exposed to environmental stress. As shown in Figure 3, at the lowest concentration of MC-LR (0.10 μg·L−1), there were no significant changes to the MDA content in rice leaves, regardless of length of exposure. After 15- and 34-days exposure to 1.0, 10.0 and 50.0 μg·L−1 MC-LR, the MDA content in rice leaves decreased significantly compared to the control (p < 0.05, p < 0.01). However, MDA concentration reduced significantly only in the 50.0 μg·L−1 group after 20 days exposure (p < 0.05).
MDA is usually an important indicator of environmentally induced oxidative stress. When rice plants experience other stresses, including cadmium, mercury, or water changes, an increase in the level of MDA is observed [37,38]. Similar to this, the rise in LPO levels is a key factor in MC-LR induced toxicity to aquatic plants is in the increase in the level of LPO. According to Jiang et al. [1], Vallisneria natans subjected to environmental concentrations of MC-LR showed considerably higher MDA content. However, the current study showed that after MC-LR exposure, the significant decreased MDA contents were observed in several groups. This observation may be related to the intimate relationship between the level of intracellular structural damage and changes in ROS levels [1].
3.5. Effects of MC-LR exposure on the activity of sucrose synthase (SS) in rice leaves
As depicted in Figure 4, the activity of SS in rice leaves significantly decreased after exposure to 1.0 and 10.0 μg·L−1 MC-LR for 7 days compared to the control (p < 0.05). The 50.0 μg·L−1 MC-LR treatment group’s SS activity in rice leaves increased significantly on day 15 compared to the control group (p < 0.05). After 20 days exposure, the activity of SS reduced significantly in comparison to the control in the 0.10 μg·L−1 MC-LR group, whereas increased significantly in the 1.0 μg·L−1 group (p < 0.05). After 34 days, the activity of SS was significantly lower in the 0.10, 10.0, and 50.0 μg·L−1 MC-LR groups in comparison to control (p < 0.05).
MCs can specifically inhibit the activity of intracellular PP1 and PP2A, thereby activating protein kinase and cyclooxygenase to promote the phosphorylation of a variety of intracellular proteins. This disrupts the equilibrium between intracellular protein phosphorylation and dephosphorylation, inhibits intracellular signal transduction, and leads to a range of other physiological and biochemical responses that ultimately result in cell damage [4,20]. In the late stage of exposure (34 days), the activity of SS saw an overall decrease compared to control, particularly in the groups exposed to 0.10,10.0, and 50.0 μg·L−1 MC-LR. Lower SS activity may influence cell differentiation and cell wall synthesis and can reduce the plants’ ability to accumulate carbon by decreasing the plants’ resistance. The result is a reduction in vegetative growth, which we have shown in this a previous study, where the rice seedlings subjected to MC-LR for 34 days showed significantly reduced plant height and fresh weight [10].
3.6. Effects of MC-LR on iNOS and TNOS in rice leaves
The activity of iNOS in the leaves of rice plants treated with MC-LR is depicted in Figure 5a. With exposure to 50.0 μg·L−1 MC-LR for 7 and 15 days, the iNOS activity was significantly increased as compared to the control (p < 0.05). After 34 days, the group treated with 0.1 μg·L−1 of MC-LR showed significantly higher iNOS activity in comparison to the control group. In contrast, 10.0 and 50.0 μg·L−1 MC-LR exposure significantly lowered the iNOS activity as compared to the control (p < 0.05, p < 0.01). There were no significant differences in TNOS activities when exposed rice to varied concentrations of MC-LR for 20 days compared to the control. The TNOS activity in plants treated with MC-LR is shown in Figure 5b. Following the exposure to 50.0 μg·L−1 MC-LR for 7 and 20 days, the TNOS activity in rice leaves was significantly induced in comparison to control (p < 0.05). After beint exposed to 0.10 μg·L−1 MC-LR for 34 days, the TNOS activity was significantly greater than the control. In contrast, the TNOS activity was significantly lower after exposure to 10.0 and 50.0 μg·L−1 MC-LR (p < 0.05, p < 0.01). There were no remarkable differences in the TNOS activities when exposed to various concentrations of MC-LR for 15 days compared to the control. A significant positive correlation was found between iNOS and TNOS activity in rice leaves under MC-LR exposure (Figure 6).
Nearly all plant physiological processes involve nitric oxide (NO), a signaling free radical, either directly or indirectly. Although the L-arginine (L-Arg)-dependent nitric oxide synthase (NOS), the enzymatic NO source in animal systems, has been well defined, it is still unclear how NO is produced enzymatically in higher plants [39]. NO interacts in different ways with ROS and may play a role as an antioxidant under certain stress conditions [25,26,27]. Moreover, NO can also modulate superoxide formation and inhibit LPO [27,40]. The reaction between NO and O2•-, can generate ONOO-, which can break down into the strongly oxidizing and strongly cytotoxic OH• and NO2 molecules that do damage to cells. Therefore, a balance between ROS and NO is very important. NO can also modulate root morphology and growth by regulating auxins when under stress from MC-LR [26,27]. Importantly, NO is mainly synthesized and released by NOS and nitrate reductase, among which iNOS plays a dominant transcriptional role and synthesizes most of the cellular NO [28]. Under stress, most of the NO production in plant tissues is dependent on the NOS pathway. Huang et al. [41] have shown that IBA induces the biosynthesis of NO by NOS in adventitious roots. Ji et al. [28] discovered in a different investigation that MC-LR can increase the iNOS protein levels in rat pancreatic tumor cell lines. Similar outcomes were reported in the current investigation. Rice leaves had significantly increased iNOS activity after being exposed to 50.0 μg·L−1 MC-LR for 7 and 15 days. Both iNOS and TNOS considerably increased in rice leaves after 34 days of exposure to a low concentration (0.10 μg·L−1) of MC-LR. After beingexposed to 10 and 50 μg·L−1 of MC-LR for 34 days, iNOS and TNOS concentrations were significantly lower, which echoes the findings of Chen et al. [13], who found that NO content was significantly reduced in rice exposed to MC-LR for 2 days. NOS also plays a positive role in dephosphorylation. NO-mediated apoptosis can also be triggered by the rapid induction of iNOS, and additionally causes DNA damage that needs to be further studied [28].
3.7. Correlation between physiological and biochemical indexes in rice
This study shows that exposure to dissolved MC-LR in culture solution results in the accumulation of toxins in rice leaves and roots, which can cause a range of physiological and biochemical indexes in plant leaves. The correlation between the changes of these physiological and biochemical indicators in rice plants are illustrated in Figure 6. After exposure, there was a significant negative correlation between GSH content in rice leaves and soluble MC-LR concentration in culture medium. Additionally, iNOS and TNOS activities exhibited a strong positive correlation with GSH content in leaves. The correlation between all the physiological and biochemical indexes in leaves investigated in the current study and the accumulation of toxin in leaves and roots was not significant. Normal cellular redox state in plants is represented by the amount of GSH content and the NOS related enzymes’ activities, and it is strongly correlated with the degree of oxidative stress in organisms under environmental stress. Similar correlations have been shown in other similar studies, e.g., MDA levels were found to be strong correlated with ROS levels in both aquatic and terrestrial plants [42,43]. In Vallisneria natans mesophyll cells exposed to MC-LR at a range of concentrations of 0.1–25.0 g·L−1, a similar result was also seen: a close relationship between MDA generation and superoxide radical levels (r = 0.817, p < 0.05) was shown [1].
4. Conclusion
The exposure to dissolved MC-LR in culture solution at 1.0–50.0 μg·L−1 results in the accumulation of toxins in rice leaves and roots, which can cause a range of physiological and biochemical responses. However, a significant accumulation trend of MC-LR in plants (BCF > 1) was only found in the lowest group (0.10 μg·L−1). During early exposure, up to day 10 after initial exposure, the levels of O2•- are significantly reduced. After 20 days exposure, the levels of O2•- increase overall across treatment groups, which may reflect the combined action of the plants’ antioxidant system and detoxification mechanisms. The initial reduction of O2•- content in rice leaves may take place through the induction of the antioxidant system and of NOS activity. MC-LR can affect the activity of iNOS and TNOS, thereby influencing NO production, indicating that NO may play a crucial role in the toxicity mechanism of MC-LR to rice. MDA content was significantly lower in plants exposed to relatively higher concentrations of MC-LR (1.0, 10.0 and 50.0 μg·L−1). This observation may be closely related to the level of free radical damage and changes in intracellular membrane structures in rice leaves. The inhibition of vegetative growth was also supported by the observation of overall decreased activity of SS. The changes of GSH contents in rice leaves could be recognized in a dualistic pattern, which showed positive correlation with the changes of iNOS and TNOS activities. Taken together, these changes of physiological and biochemical indexes imply that the toxicity consequences of prolonged exposure to low concentrations of MC-LR are more complicated and warrant more investigation in the future. In addition, there is a tremendous difference between hydroponics and soil cultivation in exposure. Therefore, methods for more closely analyzing actual rice growth conditions in order to ascertain more realistic physiological reactions in plants due to environmental stress are thus worthy of further investigation.
Author Contributions
Conceptualization, J.J. and Y.Y.; data curation, J.J. and Y.S.; investigation, J.J., Y.Y., X.L. and F.T.; methodology, Y.S. and T.L.; project administration, J.J.; writing—original draft, J.J. and Y.S.; writing—review and editing, J.J., Y.S. and F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Fundamental Research Funds for the Central Public Welfare Research Institutes (GYZX220202), the National Natural Science Foundation of China (21407056), and the National Key R&D Programme Projects of China (2023YFC3708700).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, J.L., Gu, X.Y., Song, R., Wang, X.R., Yang, L.Y., 2011. Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J. Hazard. Mater. 190, 188-196. [CrossRef]
- Jiang, J.L., Gu, X.Y., Song, R., Zhang, Q., Geng, J.J., Wang, X.R., Yang, L.Y., 2011. Time-dependent Oxidative Stress and Histopathological Alterations in Cyprinus carpio L. Exposed to Microcystin-LR. Ecotoxicology 20, 1000-1009. [CrossRef]
- Huisman, J., Codd, G.A., Paerl, H.W., Ibelings, B.W., Verspagen, J.M.H., Visser, P.M., 2018. Cyanobacterial blooms. Nat. Rev. Microbiol. 16, 471-483. [CrossRef]
- Wiegand, C., Pflugmacher, S., 2005. Ecotoxicological effects of selected cyanobacterial secondary metabolites: a short review. Toxicol. Appl. Pharm. 203(3), 201-218. [CrossRef]
- Huo, D., Gan, N.Q., Geng, R.Z., Cao, Q., Song, L.R., Yu, G.L., Li, R.H., 2021. Cyanobacterial blooms in China: diversity, distribution, and cyanotoxins. Harmful Algae 109, 102106. [CrossRef]
- Shu, X.B., Xie, L.Q., Wan, X., Yao, L., Xue, Q.J., Li, J.J., 2019. Vertical distribution characteristics of microcystin concentration in water and sediment of Meiliang Bay, Lake Taihu. J. Lake Sci. 31(4): 976-987. [CrossRef]
- Juliana, S.M.P., Giani, A., 2013. Estimating toxic cyanobacteria in a Brazilian reservoir by quantitative real-time PCR, based on the microcystin synthetase D gene. J. Appl. Phycol. 25, 1545-1554. [CrossRef]
- Zhou, Q, R., He, A, Y., Yang, W., Zhang, J., Wang, Z, H., Chen, J., 2018. Determination of microcystins in water by ultra-high-performance liquid chromatography-quadrupole-Orbitrap mass spectrometry. J. Environ. Health 35(6):524-527.
- Machado, J., Campos, A., Vasconcelos, V., Freitas, M., 2017. Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: a review of their relevance for agricultural plant quality and public health. Environ. Res. 153:191-204. [CrossRef]
- Zhang, M.M., Jiang, J.L., Zhou J.Y., Shan, Z.J., Bu, Y.Q., Xu, W.L., 2014. Effects of microcystin-LR at environmental relevant concentrations on growth and antioxidant enzymes of Oryza sativa L. at vegetative stage. J. Agro-Environ. Sci. 15, 101-108.
- Chen, S.H., Jiang, J.L., Long, T., Zhu, X.C., Zhang, H.C., Deng, S.P., Liu, R.B., 2021. Oxidative stress induced in rice suspension cells exposed to microcystin-LR at environmentally relevant concentrations, Environ. Sci. Pollut. Res. 28: 38393-38405. [CrossRef]
- Chen, J.Z., Song, L.R., Dai, J., Gan, N.Q., Liu, Z.L., 2004. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43, 393-400. [CrossRef]
- Chen, J., Han, F.X., Wang, F., Zhang, H., Shi, Z., 2012. Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotox. Environ. Safe. 76, 193-199. [CrossRef]
- Li, Q., Gu, P., Zhang, C., Luo, X., Zhang, H., Zhang, J., Zheng, Z., 2020. Combined toxic effects of anatoxin-a and microcystin-LR on submerged macrophytes and biofilms. J. Hazard. Mater. 389, 122053. [CrossRef]
- Wang, Y., Ye, H., Bai, J., Ren, F., 2021. The regulatory framework of developmentally programmed cell death in floral organs: a review. Plant Physiol. Bioch. 158:103-112. [CrossRef]
- Máthé, C., M-Hamvas, M., Vasas, G., 2013. Microcystin-LR and cylindrospermopsin induced alterations in chromatin organization of plant cells. Mar. Drugs 11(10), 3689-3717. [CrossRef]
- Tsoumalakou, E., Papadimitriou, T., Berillis, P., Kormas, K.A., Levizou, E., 2021. Spray irrigation with microcystins-rich water affects plant performance from the microscopic to the functional level and food safety of spinach (Spinacia oleracea L.). Trends Plant Sci. 27(2):116-119. Sci. Total Environ. 789, 147948. [CrossRef]
- Romero-Oliva, C.S., Contardo-Jara, V., Block, T., Pflugmacher, S., 2014. Accumulation of microcystin congeners in different aquatic plants and crops—a case study from lake Amatitlán, Guatemala. Ecotox. Environ. Safe. 102: 121-128. [CrossRef]
- Jiang, J.L., Zhang H., Long T., Li X.Z., Yang Y., Chen Q., 2022. Regulation of oxidative stress and programmed cell death related genes induced by microcystin-LR in rice suspension cells. J. Environ. Manag. [CrossRef]
- Kurki-Helasmo, K., Meriluoto, J., 1998. Microcystin uptake inhibits growth and protein phosphatase activity in Mustard (Sinapis alba L.) seedlings. Toxicon 36, 1921-1921. [CrossRef]
- Hastie, C.J., Borthwick, E.B., Morrison, L.F., Codd, G.A., Cohen, P.T.W, 2005. Inhibition of several protein phosphatases by a non-covalently interacting microcystin and a novel cyanobacterial peptide, nostocyclin. Biochim. Biophys. Acta 1726, 187-193. [CrossRef]
- Jiang, J.L., 2015. Rice plant response to long-term microcystin-LR exposure: Gene expression profiling. Jiang, J. (2015). Rice plant response to long-term microcystin-LR exposure: Gene expression profiling. Toxicol. Lett. 238. [CrossRef]
- Plugmacher, S., 2004. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol, 70, 169-178. [CrossRef]
- Thanh-Son, D., Thai-Hang, L., Thanh-Luu, P., Lan-Chi, D.H., Phuoc-Dan, N., 2014. Influences of cyanobacterial toxins microcystins on the seedling of plants. J. Environ. Prot. 5, 35-41. [CrossRef]
- Beligni, M.V., Lamattina, L., 1999. Is nitric oxide toxic or protective? Trends Plant Sci. 4, 299-299. [CrossRef]
- Yadav, S., David, A., Bhatla, S.C., 2011. Nitric oxide accumulation and actin distribution during auxin-induced adventitious root development in sunflower. Sci. Hortic. 129, 159-166. [CrossRef]
- Chen, J., Zhang, H.Q., Hu, L.B., Shi, Z.Q., 2013. Microcystin-LR-induced phytotoxicity in rice crown root is associated with the cross-talk between auxin and nitric oxide. Chemosphere 93, 283-293. [CrossRef]
- Ji, Y., Lu, G., Chen, G.Q., Huang, B., Zhang, X., Shen, K., Wu, S., 2011. Microcystin-LR induces apoptosis via NF-κB /iNOS pathway in INS-1 cells. Int. J. Mol. Sci. 12, 4722-4734. [CrossRef]
- Hissin, P.J., Hilf, R., 1976. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 74, 214-226. [CrossRef]
- Bradford, M.M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. [CrossRef]
- Meier-Abt, F., Hammann-Hänni, A., Stieger, B., Ballatoria, N., Boyer, J.L., 2007. The organic anion transport polypeptide 1d1 (Oatp1d1) mediates hepatocellular uptake of phalloidin and microcystin into skate liver. Toxicol. Appl. Pharmacol. 218, 274-279. [CrossRef]
- Yin, G., 2015. Preliminary study on accumulation and transport of microcystin MC-LR in rice. Nanjing Normal University.
- Saqrane, S., ghazali, I.E., Ouahid, Y., Hassni, M.E., Hadrami, I.E., Bouarab, L., Del Campo, F.F., Oudra, B., Vasconcelos, V., 2007. Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: microcystin accumulation, detoxication and oxidative stress induction. Aquat. Toxicol. 83, 284-294. [CrossRef]
- Maejima, K., Muraoka, T., Park, H.D., 2014. Accumulation and inhibitory effects of microcystin on the growth of rice and broccoli. KJEE 47 (Special issue): 19-30. [CrossRef]
- Stüven, J., Pflugmacher, S., 2007. Antioxidative stress response of Lepidium sativum due to exposure to cyanobacterial secondary metabolites. Toxicon 50, 85-93. [CrossRef]
- Pflugmacher, S., Wiegand, C., Oberemm, A., Beattie, K.A., Krause, E., Codd, G.A., Steinberg, C.E.W., 1999. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: the first step of detoxication. Biochem. Biophys. Acta. 1425, 527–533. [CrossRef]
- Shulaev, V., Cortes, D., Miller, G., Mittler, R., 2008. Metabolomics for plant stress response. Physiol. Plantarum 132, 199-208. [CrossRef]
- Zhang, Y.P., Sun, G., Yang, M.L., Wu, H.H., Zhang, J.Z., Song, S.J., Ma, E.B., Guo, Y.P., 2011. Chronic accumulation of cadmium and its effects on antioxidant enzymes and malondialdehyde in Oxya chinensis (Orthoptera:Acridoidea). Ecotox. Environ. Safe. 74, 1355-1362. [CrossRef]
- Corpas, F.J., González-Gordo, S., Palma, J.M., 2022. NO source in higher plants: present and future of an unresolved question. Trends Plant Sci. 27(2):116-119. [CrossRef]
- Caro, A., Puntarulo, S., 1998. Nitric oxide decreases superoxide anion generation by microsomes from soybean embryonic axes. Physiol. Plantarum.104, 357-364. [CrossRef]
- Huang, A.X., She, X.P., Huang, C., Song, T.S., 2007. The dynamic distribution of NO and NADPH-diaphorase activity during IBA-induced adventitious root formation. Physiol. Plantarum, 130, 240-249. [CrossRef]
- Wang, C. R., Wang, X. R., Tian, Y., Yu, H. X., Gu, X. Y., Du, W. C., & Zhou, H., 2008. Oxidative stress, defense response, and early biomarkers for lead-contaminated soil in Vicia faba seedlings. Environ. Toxicol. Chem. 27(4), 970-977. [CrossRef]
- Yin, Y., Wang, X., Sun, Y., Guo, H., Yin, D., 2008. Bioaccumulation and oxidative stress in submerged macrophyte Ceratophyllum demersum L. upon exposure to pyrene, Environ. Toxicol. 23(3), 328-336. [CrossRef]
Figure 1.
Levels of the superoxide anion O2•- in rice leaves after MC-LR exposure. Data are denoted as mean ± standard deviation (n = 4). Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 1.
Levels of the superoxide anion O2•- in rice leaves after MC-LR exposure. Data are denoted as mean ± standard deviation (n = 4). Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
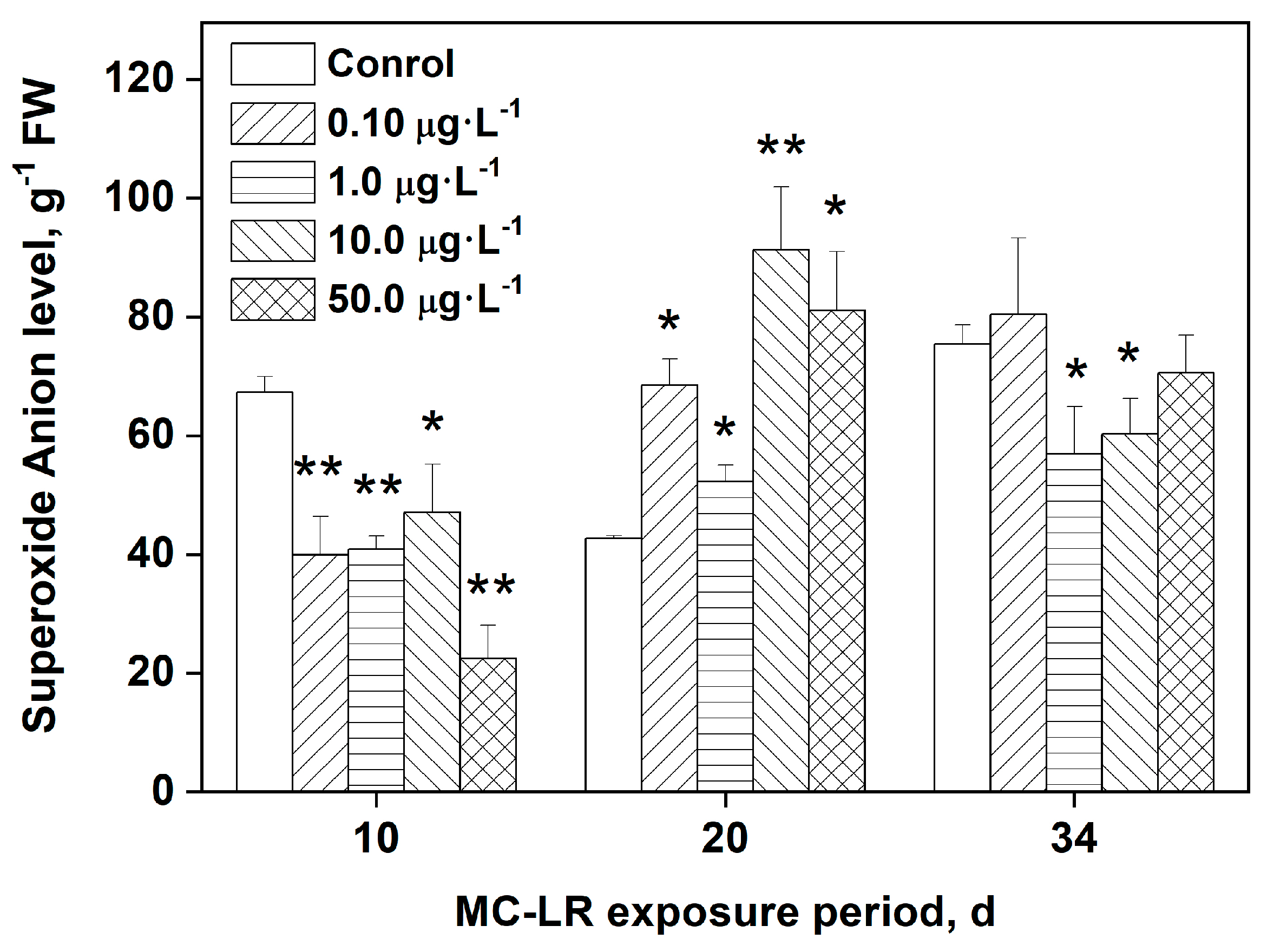
Figure 2.
Levels of GSH in the leaves of rice seedling exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 2.
Levels of GSH in the leaves of rice seedling exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 3.
Level of MDA in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 3.
Level of MDA in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 4.
Activity of SS in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 4.
Activity of SS in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 5.
Activity of iNOS (a) and TNOS (b) in the leaves of rice seedlings exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 5.
Activity of iNOS (a) and TNOS (b) in the leaves of rice seedlings exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 6.
Correlation between physiological and biochemical indexes in rice. The size of the circle and the color depth in the Correlation Heat Map represent the size of the Pearson Correlation Coefficient, Asterisk indicates the correlation is significant (p ≤ 0.05). Env-MC: MC-LR concentrations in the nutrient solution; Accum-L: MC-LR contents in rice leaves; Accum-R: MC-LR contents in rice roots.
Figure 6.
Correlation between physiological and biochemical indexes in rice. The size of the circle and the color depth in the Correlation Heat Map represent the size of the Pearson Correlation Coefficient, Asterisk indicates the correlation is significant (p ≤ 0.05). Env-MC: MC-LR concentrations in the nutrient solution; Accum-L: MC-LR contents in rice leaves; Accum-R: MC-LR contents in rice roots.

Table 1.
Bioaccumulation of MC-LR in rice leaves and roots.
| MC-LR concentration, μg/L | Bioaccumulation of MC-LR in rice leaves and roots | ||||||||||
| Leaves, μg/g FW | Roots, μg/g FW | ||||||||||
| Exposure period a, d | BCF b | Exposure period, d | BCF b | ||||||||
| 15 | 20 | 34 | 7 | 15 | 20 | 34 | |||||
| 0.1 | 0.54 ± 0.09 | 0.59 ± 0.08 | 0.51 ± 0.09 | 5.90 ± 0.80 | 0.68 ± 0.05 | 0.77 ± 0.04 | 0.75 ± 0.14 | 0.67 ± 0.05 | 7.70 ± 0.40 | ||
| 1.0 | 0.67 ± 0.06 | 0.69 ± 0.04 | 0.44 ± 0.10 | 0.69 ± 0.042 | 0.68 ± 0.05 | 0.72 ± 0.05 | 0.75 ± 0.03 | 0.71 ± 0.19 | 0.75 ± 0.03 | ||
| 10.0 | 0.85 ± 0.17 | 0.88 ± 0.11 | 0.78 ± 0.06 | 0.088 ± 0.011 | 1.12 ± 0.28 | 1.16 ± 0.16 | 0.98 ± 0.10 | 0.83 ± 0.19 | 0.12 ± 0.02 | ||
| 50.0 | n.a.c | 0.64 ± 0.46 | 0.33 ± 0.07 | 0.013 ± 0.01 | 1.55 ± 0.52 | 0.58 ± 0.05 | 0.46 ± 0.39 | 0.74 ± 0.21 | 0.03 ± 0.01 | ||
Data are denoted as mean ± standard deviation (n = 3). a. We did not collect enough leaves in 7-day experimental group for determination of MC-LR content; b. Here BCF stands for the maximum ratio of MC-LR in rice leaves or roots to the aqueous MC-LR concentration; c. n.a. = not available.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Alerts
Abstract
Irrigation with water containing a variety of microcystins (MCs) may pose potential threat to normal growth of agricultural plants. The mechanism of microcystin-LR (MC-LR) induced phytotoxicity in rice (Oryza sativa L.) at environmental concentrations is still unknown. Rice seedlings were exposed to MC-LR at concentrations of 0.10, 1.0, 10.0 and 50.0 μg·L−1 in hydroponic nutrient solutions for 7, 15, 20, and 34 days in the current study. The absorption and accumulation in leaf and root tissues, as well as a series of key physio-biochemical process changes in leaves of rice at different exposure time points were measured. Results showed that MC-LR could be detected in rice leaves and roots in all exposure groups, however, a significant accumulation trend of MC-LR in plants (BCF > 1) was only found in the lowest group (0.10 μg·L−1). The time-course study revealed a biphasic response of O2•- levels in rice leaves to the exposure of MC-LR, which was more pronounced in higher concentration groups, which could be attributed to the combined effects of antioxidant and detoxification mechanisms in rice. Exposure to 1.0 - 50.0 μg·L−1 MC-LR resulted in significant depletion of GSH and MDA contents in rice leaves at later exposure times. The changes of nitric oxide synthase (NOS) in rice leaves under MC-LR exposure were firstly investigated in the current study. Low MC-LR concentrations promoted NOS activity, whereas high concentrations inhibited NOS activity during the later exposure times, implying that NO may play a role in MC-LR toxicity in rice. Reduced sucrose synthase (SS) activities in rice exposed to MC-LR can reduce the plant's ability to accumulate carbon and thus may be directly related to the reduction in vegetative growth. These findings suggest that even at low concentrations of MC-LR, terrestrial plants' normal physiological status is disrupted, which, when combined with previous findings, helps reveal the mechanism of MC-LR-induced phytotoxicity.
Keywords:
Subject: Environmental and Earth Sciences - Environmental Science
1. Introduction
In recent years, numerous evidence has shown that the harmful cyanobacterial blooms are rising in frequency, magnitude and duration across the world [1,2,3]. Cyanobacterial blooms have influenced worldwide freshwater ecosystems during the last few decades. Common taxa in the bloom-forming cyanobacteria species (BFCS) include species of Microcystis, Anabaena, Nodularia, and Cylindrospermopsis, etc. [3,4,5]. As the most common BFCS, Microcystis producing toxic secondary metabolites as microcystins (MCs), has received the most attention and research. MCs are potent hepatotoxins with over 90 different variants [1,6], the most toxic and prevalent of which are microcystin-LR (MC-LR), MC-RR, and MC-YR [7,8]. During bloom outbreaks, dissolved MCs concentrations in water typically range between 0.1 and 10 μg·L−1, while ecological risks of the low MCs concentrations in natural environments remain unclear, and the risks that associated with MCs in the food chain have not been fully elucidated [1,2,9]. Moreover, MCs can enter farmland via irrigation water and will inevitably harm terrestrial plants [10,11], however, very little known about the mechanism of MC-induced phytotoxicity on terrestrial plants [12,13,14,15].
Current research on the toxic effects and mechanisms of MCs toxicity is primarily concerned with their effects on animals and human health. MCs fate in natural environments can lead to its transfer into aquatic or terrestrial organisms [9,16]. Previous research has already shown that MCs can be transferred into terrestrial plants by farmland irrigation, e.g., spray irrigation, and thus may accumulate the toxins in the plant body [17]. MC contents in crop products (Capsicum annuum and Solanum lycopersicum) irrigated with water contaminated by cyanobacterial blooms were found to be as high as 8.10 ± 4.67 μg·kg−1 DW (dry weight) [18]. Chen found that MC-LR concentrations in rice seed samples collected in the Lake Taihu area ranged from 0.04 to 3.19 μg·kg−1 [13]. Uptake and accumulation of cyanobacterial toxins in plants will inevitably trigger a series of physiological and biochemical reactions that can inhibit plant growth, causing the reduction of the quality and productivity of terrestrial agricultural plants [19].
A widely acceptable mechanism MCs specifically inhibits protein phosphatases 1 and 2A in plant and animal cells, which leads to a series of toxic downstream effects [20,21,22]. The toxicity mechanism mediated by oxidative stress in plants has also been well characterized in recent years [1,9,15,23]. Exposure to 0.50–10.0 μg·L−1 of MCs in the form of cyanobacteria crude extract or pure toxin could induce the responses of antioxidant systems of plants, as evidenced by significant changes in the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione peroxidase (GPX), and glutathione S-transferase (GST) [24]. Jiang et al. [1] also demonstrated that GSH was involved in the detoxification of MC-LR in Vallisneria natans, and oxidative damage induced in plant was demonstrated by a significant increase in the malondialdehyde (MDA) content at 1.0 μg·L−1 of dissolved MC-LR. It should be emphasized that previous studies on MCs exposure have often employed far greater concentrations that what plants really face in their environment. Nitric oxide (NO) interacts in different ways with ROS (reactive oxygen species) and may play a role as an antioxidant under certain stress conditions [25,26,27]. NO is mainly synthesized and released by nitric oxide synthase (NOS) and nitrate reductase, among which iNOS plays a dominant transcriptional role and synthesizes most of the cellular NO [28]. So far the relationship between MC-LR exposure and NO generation has not been reported in plants.
Current research on the impact of environmental concentrations of MC-LR accumulation in terrestrial plants is not extensive, nor is the research on the MCs induced physiological and biochemical responses in plants. Previous research has demonstrated that low concentrations of MC-LR promote the increase of rice biomass (including plant height, root length, and fresh weight), while at higher concentrations, MC-LR plays an inhibitory role on vegetative growth, leaving the plants small, with yellow leaves [10]. The current work aims delve further into the characteristics of absorption and accumulation in leaf and root tissues, as well as a series of key physio-biochemical process changes in leaves of rice seedlings under long-term exposure of MC-LR.
2. Materials and Methods
2.1. Chemicals and reagents
Microcystin-LR, ≥ 95% in purity (HPLC) was purchased from Taiwan algae Institute Ltd. (Taiwan Algal Science Inc). Microcystin Plate Kit was purchased from Institute of Hydrobiology, Chinese Academy of Sciences. Phenylmethylsulfonyl fluoride (PMSF), o-phthaldialdehyde, bovine serum albumin (BSA), polyvinylpolypyrrolidone (PVPP), and α-naphthylamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, acetic acid, and dimethyl sulfoxide (DMSF) was purchased from Merck (Darmstadt, Germany). Other reagents are of analytical grade and were purchased from Chinese companies. All solutions were formulated with Milli-Q water.
2.2. Experimental rice and MC-LR exposure
The Nipponbare rice variety (Oryza sativa L.) was used for the following experiments. One thousand robust and full Nipponbare rice seeds were selected and divided into four groups. The seeds were soaked at 28ºC for 24 hours and germination was induced by covering the rice seeds with three layers of wet gauze and placing them into containers lined with aluminum foil, so that the seeds would be in a dark and humid environment. The container was placed in a 25ºC incubator to induce germination and the seeds were kept in a humid environment throughout the germination process. Rice seedlings were transferred to the International Rice Research Institute in conventional nutrient solution after germination (containing 40 mg·L−1 Na+, 40 mg·L−1 K+, 40 mg·L−1 Ca2+, 40 mg·L−1 Mg2+, 10 mg·L−1 P5+, 2 mg·L−1 Fe3+, 0.5 mg·L−1 Mn2+, 0.05 mg·L−1 Mo6+, 0.2 mg·L−1 B3+, 0.01 mg·L−1 Zn2+, and 0.01 mg·L−1 Cu2+) and cultured.
Plant seedlings were cultured in nutrient solution until 5 days after germination. Plants that were growing well were selected, cleaned with ultrapure water, and subjected to the MC-LR exposure test. The test groups were exposed to MC-LR concentrations of 0.10, 1.00, 10.0, and 50.0 μg·L−1, while a negative control group was exposed to nutrient solution without MC-LR. All seedlings were exposed for 7, 15, 20, or 34 days and four parallel subgroups were defined in each group. During the exposure period, the nutrient solution containing MC-LR was replaced every other day. The initial solution was 100 ml of diluted nutrient solution, but with plant growth, the dilution was gradually reduced and the nutrient solution was increased to 200 mL to ensure that the plants had sufficient nutrients for growth. At the end of the exposure period, the plant biomass was measured immediately. Fresh leaves and roots were taken, quick-frozen under liquid nitrogen, packaged, and kept in a -80ºC freezer.
2.3. Microcystin analysis
The accumulated MC-LR in plant samples was analyzed by ELISA method with Microcystin Plate Kit according to Jiang et al. [1].
2.4. Determination of superoxide anion (O2•-) Content
On days 10, 20, and 34 after exposure to MC-LR, about 0.2 g fresh leaves were collected and accurately weighed, then quickly pulverized to powder after under liquid nitrogen. A five-fold volume of 50 mmol·L−1 phosphate buffer (pH 7.8) containing 0.1 mmol·L−1 EDTA, 4% (W/V) PVPP, and 0.3% (W/V) Triton X-100 was added to make a homogenate, which was centrifuged at 10 500×g for 20 min. Immediately, 0.5 mL of the supernatant was drawn and added to 0.5 mL of phosphate buffer (50 mmol·L−1, pH 7.8) and 1 mL of 1.0 mmol·L−1 hydroxylamine hydrochloride. The mixture was shook to mix before being incubated at 25ºC for 1 hour. One milliliter of 17.0 mmol·L−1 amino acid (prepared with glacial acetic acid and water in a 3:1 ratio) and 1 mL of 7.0 mmol/L α-naphthylamine (prepared with glacial acetic acid and water in a 3:1 ratio) were added. After mixing, the sample was incubated at 25ºC for 20 minutes before its absorbance at 530 nm was measured. Using a solution of sodium nitrite, a standard curve was generated as described above, and the O2•- content was calculated based on the standard curve.
2.5. Glutathione and malondialdehyde determination
The amounts of GSH in plant leaves were measured according to Hissin and Hilf [29] with modifications according to Jiang et al. [1]. The amounts of MDA in plant leaves were determined using a biochemical assay kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
2.6. Enzyme extraction and activity assays
About 0.2 g fresh leaves were collected and quickly pulverized to a fine powder under liquid nitrogen. The extraction of crude enzyme from the powder was according to Jiang et al. [1]. The extracts was separated into aliquots and kept at 80°C for additional investigation. All operations were carried out at 0–4oC. The determination of sucrose synthase (SS, EC 2.4.1.13), inducible nitric oxide synthase (iNOS), and total nitric oxide synthase (TNOS, EC 1.14.13.39) activities was using a commercialized biochemical assay kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. The Bradford method [30] was used in every instance to determine the total protein (Pr) level using bovine serum albumin as the reference.
2.7. Data analysis
Data were checked for normality using the Shapiro-Wilk test, and when necessary, they were converted to adhere to the normal distribution assumption. With the use of SPSS 15.0 software, a one-way ANOVA test and Duncan’s multiple range test were employed to examine the statistical significance. Differences of p < 0.05 were considered significant, while p < 0.01 were considered high significant. Quadruplicate analyses of each sample were conducted. The Pearson Correlation Coefficient between the two variables was calculated using the Correlation Plot tool in Origin 2021 and the correlation heat map was drawn.
3. Results and discussion
3.1. Levels of MC-LR in rice leaves and roots
Using enzyme-linked immunosorbent assay (ELISA), accumulated MC-LR was measured in rice leaves. For the 7-day experimental group and the 50.0 μg·L−1 MC-LR group for 15 days, we did not collect enough leaves to determine the MC-LR content. MC-LR was detected in leaf and root tissues of all exposed plants groups, as shown in Table 1. By day 20, the level of MC-LR in leaf tissues increased with increasing environmental concentration. At a given exposure concentration of MC-LR, there is an initial increase in the MC-LR detected in rice leaves and then followed by a decrease in general. The amount of MC-LR in rice roots was positively correlated with the concentration of MC-LR in the nutrient solution after exposure for 7 days (r = 0.917). We did not see a linear relationship between the exposure concentration and the amount of MC-LR in rice roots at subsequent time periods. The amount of accumulated MC-LR in rice roots decreased with time in the 10.0 μg·L−1 MC-LR group. After 15 days of treatment, the group subjected to 50.0 μg·L−1 MC-LR had considerably reduced MC-LR levels in leaves and roots as compared to exposure to lower concentrations of MC-LR.
This study calculated the relative accumulation of MC-LR in the rice roots and leaves. According to Table 1, the ratio of MC-LR in plant tissues (leaves or roots) to water in the lowest concentration treatment groups (0.1 μg·L−1) were both greater than 1, suggesting that the rice would uptake and accumulate the MC-LR in leaves and roots. However, the ratios in the treatment groups with higher concentration (10.0, 50.0 mg·kg−1) were much lower than 1, indicating that the living rice did not actively uptake a significant amount of MC-LR for self-defense.
In ecosystems, MC shows bioaccumulation in zooplankton, molluscs and crustaceans, fish, and other common invertebrates and vertebrates. Animal cells have special membrane transport proteins that play a part in toxin accumulation [31], but a transporter protein has not been confirmed for MC-LR in terrestrial plants. Recent research has explored how OsOATPM aids in the exocytosis of MC-LR in plants [19,32]. Several reports on the bioaccumulation of MCs in aquatic plants (Lemna gibba and Vallisneria natans) suggests that terrestrial plants, including food crops, can also accumulate MC through irrigation water [1,33]. By irrigating with eutrophic water containing cyanobacterial blooms and dissolved toxins, terrestrial plants may become exposed to cyanobacterial toxins. Chen et al. [12] exposed rice to 24 μg·L−1 MC-LR for 10 days and did not detect any MC in rice leaves, while the same concentration resulted in higher bioaccumulation in exposed rapeseed plant leaves. Maejima et al. [34] found that broccoli plants exposed to 0.01–10 μg·mL−1 MC-RR for 7 days did not accumulate MC-RR if they were exposed to concentrations lower than 0.1 μg·mL−1. The bioaccumulation in broccoli exposed to 1, 5, and 10 μg·mL−1 of MC-RR was 14.5, 88.9, and 145 ng·mL−1, respectively. Moreover, MC-LR was discovered for the first time to be bioaccumulated in rice grain in the Taihu Lake region, where environmental MC-LR concentrations were low and did not pose a threat to drinking water safety [13]. According to our study, the bioaccumulation of MC-LR in rice leaves and roots does not follow a linear relationship with environmental MC-LR concentrations or time of exposure. Our results show that after the same period, the MC-LR in plants tissues increases with environmental concentration, but there is a sudden decrease in MC-LR in the high concentration exposure group (50.0 μg·L−1). The BCF values of toxin in leaves and roots indicated that the MC-LR would bioaccumulate in the plant tissues and roots only happened in the lowest concentration treatment groups (0.10 μg·L−1). The MC-LR bioaccumulation, exposure concentration, exposure time, and plant type are all interrelated. When the plant exposed to a higher concentrations of MC-LR, its defense mechanisms are activated. The bioaccumulated MC-LR in rice might be partially bio-transformed through a glutathione-related pathway, which was also the important detoxification pathway in aquatic plants [4,23]. The cell structure damages in plant cells caused by high concentrations of MC-LR exposure may also lead to decreased MC-LR accumulation [19,35].
3.2. Effects of MC-LR exposure on O2•- levels in rice leaves
ROS are often associated with environmental stress-related plant toxicity. Figure 1 depicts the levels of O2•- in rice leaves following MC-LR exposure. During early exposure times, the rice plants are still in the seedling stage and provide inadequate sample sizes. As a result, we began measuring the levels of O2•- after 10 days of MC-LR exposure, which was also more reflective of changes in enzymatic activities. As shown in Figure 1, after 10 days of MC-LR exposure, all groups had significantly lower levels of O2•- than the control group (p < 0.05, p < 0.01). The levels of O2•- in the treatment groups, on the other hand, were significantly higher than those in the control group (p < 0.05, p < 0.01) after 20 days of exposure, with increases of 60.7%, 27.7%, 114%, and 90.1%, respectively. By day 34, overall O2•- levels appear to be decreasing, with O2•- levels in the 1.0 and 10.0 μg·L−1 MC-LR even significantly lower when compared to the control group (p < 0.05).
ROS are produced in vivo under normal physiological conditions, reacting chemically with biological macromolecules, including unsaturated fatty acids, proteins, and DNA [1,2,35]. These reactions result in damage to macromolecules, which affect their normal physiological functions and induce oxidative stress. Under normal physiological conditions, there is a dynamic equilibrium between the ROS production and the antioxidant defense system of the organism. MCs have been shown in studies to directly induce the excessive production of ROS in aquatic plant tissues, and the ROS levels in a plant are closely related to environmental stress-induced toxicity [1,23]. The results of our study demonstrated that MC-LR indeed directly induces excessive O2•- production in rice leaves. As shown in Figure 1, after 10 days of MC-LR exposure, the concentration of O2•- decreased with increasing MC-LR concentrations, reaching a minimum when exposed to 50.0 μg·L−1 MC-LR. This is likely due to the synthesis results of the early responses of antioxidant system and damages of important subcellular structures caused by MC-LR exposure, e.g., cell membrane of mesophyll cells [1]. After 20 days of exposure, plants exposed to 0.10,10.0, and 50.0 μg·L−1 MC-LR had significantly higher O2•- levels in their leaves, indicating that the metabolism reaction and detoxification conversion of MC-LR occurred, leading to the formation of excessive ROS. Similarly, Chen et al. [13] found that 2 mg·L−1 MC-LR exposure for 2 days results in significantly higher levels of ROS in the rice root system compared to the control.
3.3. Effects of MC-LR exposure on GSH levels in rice leaves
Figure 2 depicts the changes of GSH content in rice leaves following MC-LR treatment. The GSH content in rice leaves was significantly greater after 7 days of exposure to 50.0 μg·L−1 MC-LR compared to the control (p < 0.05). On the contrary, exposure to 50.0 μg·L−1 MC-LR for 15, 20, and 34 days resulted in significantly reduced GSH levels in rice leaves as compared to the control (p < 0.05, p < 0.01). Furthermore, exposure to 0.10 μg·L−1 MC-LR for 34 days significantly increased the GSH content in rice leaves, whereas exposure to higher MC-LR concentrations of 1.0 and 10.0 μg·L−1 resulted in a significant reduction in GSH.
Numerous studies have demonstrated through in vitro cell-free systems or in vivo that, as the most abundant cellular thiol involved in the removal of ROS, GSH also plays an important role in the detoxification of MCs [1,36]. In the present study, there was a significant decrease in GSH content in the leaves of rice plants exposed to high concentrations of MC-LR after 15, 20, and 34 days of treatment (50.0 μg·L−1), indicating that GSH is involved in MC-LR detoxification, most likely by binding to MC-LR, increasing its solubility, and aiding in its removal from the organism. It is also possible that a higher concentration of MC-LR inhibits the synthesis of GSH. In addition, oxidative stress causes GSH to convert to the oxidized GSSG form, resulting in a decrease of GSH. Therefore, there are many possible reasons behind the changes in GSH content. As shown in Figure 2, after undergoing MC-LR stress for 34 days, the toxin at low concentration (0.10 μg·L−1) can also stimulate the synthesis of GSH. Thus, changes in GSH content are the result of a comprehensive systemic response.
3.4. Effects of MC-LR exposure on MDA levels in rice leaves
As a marker for lipid peroxidation, the intracellular MDA content changes when the plant is exposed to environmental stress. As shown in Figure 3, at the lowest concentration of MC-LR (0.10 μg·L−1), there were no significant changes to the MDA content in rice leaves, regardless of length of exposure. After 15- and 34-days exposure to 1.0, 10.0 and 50.0 μg·L−1 MC-LR, the MDA content in rice leaves decreased significantly compared to the control (p < 0.05, p < 0.01). However, MDA concentration reduced significantly only in the 50.0 μg·L−1 group after 20 days exposure (p < 0.05).
MDA is usually an important indicator of environmentally induced oxidative stress. When rice plants experience other stresses, including cadmium, mercury, or water changes, an increase in the level of MDA is observed [37,38]. Similar to this, the rise in LPO levels is a key factor in MC-LR induced toxicity to aquatic plants is in the increase in the level of LPO. According to Jiang et al. [1], Vallisneria natans subjected to environmental concentrations of MC-LR showed considerably higher MDA content. However, the current study showed that after MC-LR exposure, the significant decreased MDA contents were observed in several groups. This observation may be related to the intimate relationship between the level of intracellular structural damage and changes in ROS levels [1].
3.5. Effects of MC-LR exposure on the activity of sucrose synthase (SS) in rice leaves
As depicted in Figure 4, the activity of SS in rice leaves significantly decreased after exposure to 1.0 and 10.0 μg·L−1 MC-LR for 7 days compared to the control (p < 0.05). The 50.0 μg·L−1 MC-LR treatment group’s SS activity in rice leaves increased significantly on day 15 compared to the control group (p < 0.05). After 20 days exposure, the activity of SS reduced significantly in comparison to the control in the 0.10 μg·L−1 MC-LR group, whereas increased significantly in the 1.0 μg·L−1 group (p < 0.05). After 34 days, the activity of SS was significantly lower in the 0.10, 10.0, and 50.0 μg·L−1 MC-LR groups in comparison to control (p < 0.05).
MCs can specifically inhibit the activity of intracellular PP1 and PP2A, thereby activating protein kinase and cyclooxygenase to promote the phosphorylation of a variety of intracellular proteins. This disrupts the equilibrium between intracellular protein phosphorylation and dephosphorylation, inhibits intracellular signal transduction, and leads to a range of other physiological and biochemical responses that ultimately result in cell damage [4,20]. In the late stage of exposure (34 days), the activity of SS saw an overall decrease compared to control, particularly in the groups exposed to 0.10,10.0, and 50.0 μg·L−1 MC-LR. Lower SS activity may influence cell differentiation and cell wall synthesis and can reduce the plants’ ability to accumulate carbon by decreasing the plants’ resistance. The result is a reduction in vegetative growth, which we have shown in this a previous study, where the rice seedlings subjected to MC-LR for 34 days showed significantly reduced plant height and fresh weight [10].
3.6. Effects of MC-LR on iNOS and TNOS in rice leaves
The activity of iNOS in the leaves of rice plants treated with MC-LR is depicted in Figure 5a. With exposure to 50.0 μg·L−1 MC-LR for 7 and 15 days, the iNOS activity was significantly increased as compared to the control (p < 0.05). After 34 days, the group treated with 0.1 μg·L−1 of MC-LR showed significantly higher iNOS activity in comparison to the control group. In contrast, 10.0 and 50.0 μg·L−1 MC-LR exposure significantly lowered the iNOS activity as compared to the control (p < 0.05, p < 0.01). There were no significant differences in TNOS activities when exposed rice to varied concentrations of MC-LR for 20 days compared to the control. The TNOS activity in plants treated with MC-LR is shown in Figure 5b. Following the exposure to 50.0 μg·L−1 MC-LR for 7 and 20 days, the TNOS activity in rice leaves was significantly induced in comparison to control (p < 0.05). After beint exposed to 0.10 μg·L−1 MC-LR for 34 days, the TNOS activity was significantly greater than the control. In contrast, the TNOS activity was significantly lower after exposure to 10.0 and 50.0 μg·L−1 MC-LR (p < 0.05, p < 0.01). There were no remarkable differences in the TNOS activities when exposed to various concentrations of MC-LR for 15 days compared to the control. A significant positive correlation was found between iNOS and TNOS activity in rice leaves under MC-LR exposure (Figure 6).
Nearly all plant physiological processes involve nitric oxide (NO), a signaling free radical, either directly or indirectly. Although the L-arginine (L-Arg)-dependent nitric oxide synthase (NOS), the enzymatic NO source in animal systems, has been well defined, it is still unclear how NO is produced enzymatically in higher plants [39]. NO interacts in different ways with ROS and may play a role as an antioxidant under certain stress conditions [25,26,27]. Moreover, NO can also modulate superoxide formation and inhibit LPO [27,40]. The reaction between NO and O2•-, can generate ONOO-, which can break down into the strongly oxidizing and strongly cytotoxic OH• and NO2 molecules that do damage to cells. Therefore, a balance between ROS and NO is very important. NO can also modulate root morphology and growth by regulating auxins when under stress from MC-LR [26,27]. Importantly, NO is mainly synthesized and released by NOS and nitrate reductase, among which iNOS plays a dominant transcriptional role and synthesizes most of the cellular NO [28]. Under stress, most of the NO production in plant tissues is dependent on the NOS pathway. Huang et al. [41] have shown that IBA induces the biosynthesis of NO by NOS in adventitious roots. Ji et al. [28] discovered in a different investigation that MC-LR can increase the iNOS protein levels in rat pancreatic tumor cell lines. Similar outcomes were reported in the current investigation. Rice leaves had significantly increased iNOS activity after being exposed to 50.0 μg·L−1 MC-LR for 7 and 15 days. Both iNOS and TNOS considerably increased in rice leaves after 34 days of exposure to a low concentration (0.10 μg·L−1) of MC-LR. After beingexposed to 10 and 50 μg·L−1 of MC-LR for 34 days, iNOS and TNOS concentrations were significantly lower, which echoes the findings of Chen et al. [13], who found that NO content was significantly reduced in rice exposed to MC-LR for 2 days. NOS also plays a positive role in dephosphorylation. NO-mediated apoptosis can also be triggered by the rapid induction of iNOS, and additionally causes DNA damage that needs to be further studied [28].
3.7. Correlation between physiological and biochemical indexes in rice
This study shows that exposure to dissolved MC-LR in culture solution results in the accumulation of toxins in rice leaves and roots, which can cause a range of physiological and biochemical indexes in plant leaves. The correlation between the changes of these physiological and biochemical indicators in rice plants are illustrated in Figure 6. After exposure, there was a significant negative correlation between GSH content in rice leaves and soluble MC-LR concentration in culture medium. Additionally, iNOS and TNOS activities exhibited a strong positive correlation with GSH content in leaves. The correlation between all the physiological and biochemical indexes in leaves investigated in the current study and the accumulation of toxin in leaves and roots was not significant. Normal cellular redox state in plants is represented by the amount of GSH content and the NOS related enzymes’ activities, and it is strongly correlated with the degree of oxidative stress in organisms under environmental stress. Similar correlations have been shown in other similar studies, e.g., MDA levels were found to be strong correlated with ROS levels in both aquatic and terrestrial plants [42,43]. In Vallisneria natans mesophyll cells exposed to MC-LR at a range of concentrations of 0.1–25.0 g·L−1, a similar result was also seen: a close relationship between MDA generation and superoxide radical levels (r = 0.817, p < 0.05) was shown [1].
4. Conclusion
The exposure to dissolved MC-LR in culture solution at 1.0–50.0 μg·L−1 results in the accumulation of toxins in rice leaves and roots, which can cause a range of physiological and biochemical responses. However, a significant accumulation trend of MC-LR in plants (BCF > 1) was only found in the lowest group (0.10 μg·L−1). During early exposure, up to day 10 after initial exposure, the levels of O2•- are significantly reduced. After 20 days exposure, the levels of O2•- increase overall across treatment groups, which may reflect the combined action of the plants’ antioxidant system and detoxification mechanisms. The initial reduction of O2•- content in rice leaves may take place through the induction of the antioxidant system and of NOS activity. MC-LR can affect the activity of iNOS and TNOS, thereby influencing NO production, indicating that NO may play a crucial role in the toxicity mechanism of MC-LR to rice. MDA content was significantly lower in plants exposed to relatively higher concentrations of MC-LR (1.0, 10.0 and 50.0 μg·L−1). This observation may be closely related to the level of free radical damage and changes in intracellular membrane structures in rice leaves. The inhibition of vegetative growth was also supported by the observation of overall decreased activity of SS. The changes of GSH contents in rice leaves could be recognized in a dualistic pattern, which showed positive correlation with the changes of iNOS and TNOS activities. Taken together, these changes of physiological and biochemical indexes imply that the toxicity consequences of prolonged exposure to low concentrations of MC-LR are more complicated and warrant more investigation in the future. In addition, there is a tremendous difference between hydroponics and soil cultivation in exposure. Therefore, methods for more closely analyzing actual rice growth conditions in order to ascertain more realistic physiological reactions in plants due to environmental stress are thus worthy of further investigation.
Author Contributions
Conceptualization, J.J. and Y.Y.; data curation, J.J. and Y.S.; investigation, J.J., Y.Y., X.L. and F.T.; methodology, Y.S. and T.L.; project administration, J.J.; writing—original draft, J.J. and Y.S.; writing—review and editing, J.J., Y.S. and F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Fundamental Research Funds for the Central Public Welfare Research Institutes (GYZX220202), the National Natural Science Foundation of China (21407056), and the National Key R&D Programme Projects of China (2023YFC3708700).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, J.L., Gu, X.Y., Song, R., Wang, X.R., Yang, L.Y., 2011. Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J. Hazard. Mater. 190, 188-196. [CrossRef]
- Jiang, J.L., Gu, X.Y., Song, R., Zhang, Q., Geng, J.J., Wang, X.R., Yang, L.Y., 2011. Time-dependent Oxidative Stress and Histopathological Alterations in Cyprinus carpio L. Exposed to Microcystin-LR. Ecotoxicology 20, 1000-1009. [CrossRef]
- Huisman, J., Codd, G.A., Paerl, H.W., Ibelings, B.W., Verspagen, J.M.H., Visser, P.M., 2018. Cyanobacterial blooms. Nat. Rev. Microbiol. 16, 471-483. [CrossRef]
- Wiegand, C., Pflugmacher, S., 2005. Ecotoxicological effects of selected cyanobacterial secondary metabolites: a short review. Toxicol. Appl. Pharm. 203(3), 201-218. [CrossRef]
- Huo, D., Gan, N.Q., Geng, R.Z., Cao, Q., Song, L.R., Yu, G.L., Li, R.H., 2021. Cyanobacterial blooms in China: diversity, distribution, and cyanotoxins. Harmful Algae 109, 102106. [CrossRef]
- Shu, X.B., Xie, L.Q., Wan, X., Yao, L., Xue, Q.J., Li, J.J., 2019. Vertical distribution characteristics of microcystin concentration in water and sediment of Meiliang Bay, Lake Taihu. J. Lake Sci. 31(4): 976-987. [CrossRef]
- Juliana, S.M.P., Giani, A., 2013. Estimating toxic cyanobacteria in a Brazilian reservoir by quantitative real-time PCR, based on the microcystin synthetase D gene. J. Appl. Phycol. 25, 1545-1554. [CrossRef]
- Zhou, Q, R., He, A, Y., Yang, W., Zhang, J., Wang, Z, H., Chen, J., 2018. Determination of microcystins in water by ultra-high-performance liquid chromatography-quadrupole-Orbitrap mass spectrometry. J. Environ. Health 35(6):524-527.
- Machado, J., Campos, A., Vasconcelos, V., Freitas, M., 2017. Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: a review of their relevance for agricultural plant quality and public health. Environ. Res. 153:191-204. [CrossRef]
- Zhang, M.M., Jiang, J.L., Zhou J.Y., Shan, Z.J., Bu, Y.Q., Xu, W.L., 2014. Effects of microcystin-LR at environmental relevant concentrations on growth and antioxidant enzymes of Oryza sativa L. at vegetative stage. J. Agro-Environ. Sci. 15, 101-108.
- Chen, S.H., Jiang, J.L., Long, T., Zhu, X.C., Zhang, H.C., Deng, S.P., Liu, R.B., 2021. Oxidative stress induced in rice suspension cells exposed to microcystin-LR at environmentally relevant concentrations, Environ. Sci. Pollut. Res. 28: 38393-38405. [CrossRef]
- Chen, J.Z., Song, L.R., Dai, J., Gan, N.Q., Liu, Z.L., 2004. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43, 393-400. [CrossRef]
- Chen, J., Han, F.X., Wang, F., Zhang, H., Shi, Z., 2012. Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotox. Environ. Safe. 76, 193-199. [CrossRef]
- Li, Q., Gu, P., Zhang, C., Luo, X., Zhang, H., Zhang, J., Zheng, Z., 2020. Combined toxic effects of anatoxin-a and microcystin-LR on submerged macrophytes and biofilms. J. Hazard. Mater. 389, 122053. [CrossRef]
- Wang, Y., Ye, H., Bai, J., Ren, F., 2021. The regulatory framework of developmentally programmed cell death in floral organs: a review. Plant Physiol. Bioch. 158:103-112. [CrossRef]
- Máthé, C., M-Hamvas, M., Vasas, G., 2013. Microcystin-LR and cylindrospermopsin induced alterations in chromatin organization of plant cells. Mar. Drugs 11(10), 3689-3717. [CrossRef]
- Tsoumalakou, E., Papadimitriou, T., Berillis, P., Kormas, K.A., Levizou, E., 2021. Spray irrigation with microcystins-rich water affects plant performance from the microscopic to the functional level and food safety of spinach (Spinacia oleracea L.). Trends Plant Sci. 27(2):116-119. Sci. Total Environ. 789, 147948. [CrossRef]
- Romero-Oliva, C.S., Contardo-Jara, V., Block, T., Pflugmacher, S., 2014. Accumulation of microcystin congeners in different aquatic plants and crops—a case study from lake Amatitlán, Guatemala. Ecotox. Environ. Safe. 102: 121-128. [CrossRef]
- Jiang, J.L., Zhang H., Long T., Li X.Z., Yang Y., Chen Q., 2022. Regulation of oxidative stress and programmed cell death related genes induced by microcystin-LR in rice suspension cells. J. Environ. Manag. [CrossRef]
- Kurki-Helasmo, K., Meriluoto, J., 1998. Microcystin uptake inhibits growth and protein phosphatase activity in Mustard (Sinapis alba L.) seedlings. Toxicon 36, 1921-1921. [CrossRef]
- Hastie, C.J., Borthwick, E.B., Morrison, L.F., Codd, G.A., Cohen, P.T.W, 2005. Inhibition of several protein phosphatases by a non-covalently interacting microcystin and a novel cyanobacterial peptide, nostocyclin. Biochim. Biophys. Acta 1726, 187-193. [CrossRef]
- Jiang, J.L., 2015. Rice plant response to long-term microcystin-LR exposure: Gene expression profiling. Jiang, J. (2015). Rice plant response to long-term microcystin-LR exposure: Gene expression profiling. Toxicol. Lett. 238. [CrossRef]
- Plugmacher, S., 2004. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol, 70, 169-178. [CrossRef]
- Thanh-Son, D., Thai-Hang, L., Thanh-Luu, P., Lan-Chi, D.H., Phuoc-Dan, N., 2014. Influences of cyanobacterial toxins microcystins on the seedling of plants. J. Environ. Prot. 5, 35-41. [CrossRef]
- Beligni, M.V., Lamattina, L., 1999. Is nitric oxide toxic or protective? Trends Plant Sci. 4, 299-299. [CrossRef]
- Yadav, S., David, A., Bhatla, S.C., 2011. Nitric oxide accumulation and actin distribution during auxin-induced adventitious root development in sunflower. Sci. Hortic. 129, 159-166. [CrossRef]
- Chen, J., Zhang, H.Q., Hu, L.B., Shi, Z.Q., 2013. Microcystin-LR-induced phytotoxicity in rice crown root is associated with the cross-talk between auxin and nitric oxide. Chemosphere 93, 283-293. [CrossRef]
- Ji, Y., Lu, G., Chen, G.Q., Huang, B., Zhang, X., Shen, K., Wu, S., 2011. Microcystin-LR induces apoptosis via NF-κB /iNOS pathway in INS-1 cells. Int. J. Mol. Sci. 12, 4722-4734. [CrossRef]
- Hissin, P.J., Hilf, R., 1976. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 74, 214-226. [CrossRef]
- Bradford, M.M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. [CrossRef]
- Meier-Abt, F., Hammann-Hänni, A., Stieger, B., Ballatoria, N., Boyer, J.L., 2007. The organic anion transport polypeptide 1d1 (Oatp1d1) mediates hepatocellular uptake of phalloidin and microcystin into skate liver. Toxicol. Appl. Pharmacol. 218, 274-279. [CrossRef]
- Yin, G., 2015. Preliminary study on accumulation and transport of microcystin MC-LR in rice. Nanjing Normal University.
- Saqrane, S., ghazali, I.E., Ouahid, Y., Hassni, M.E., Hadrami, I.E., Bouarab, L., Del Campo, F.F., Oudra, B., Vasconcelos, V., 2007. Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: microcystin accumulation, detoxication and oxidative stress induction. Aquat. Toxicol. 83, 284-294. [CrossRef]
- Maejima, K., Muraoka, T., Park, H.D., 2014. Accumulation and inhibitory effects of microcystin on the growth of rice and broccoli. KJEE 47 (Special issue): 19-30. [CrossRef]
- Stüven, J., Pflugmacher, S., 2007. Antioxidative stress response of Lepidium sativum due to exposure to cyanobacterial secondary metabolites. Toxicon 50, 85-93. [CrossRef]
- Pflugmacher, S., Wiegand, C., Oberemm, A., Beattie, K.A., Krause, E., Codd, G.A., Steinberg, C.E.W., 1999. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: the first step of detoxication. Biochem. Biophys. Acta. 1425, 527–533. [CrossRef]
- Shulaev, V., Cortes, D., Miller, G., Mittler, R., 2008. Metabolomics for plant stress response. Physiol. Plantarum 132, 199-208. [CrossRef]
- Zhang, Y.P., Sun, G., Yang, M.L., Wu, H.H., Zhang, J.Z., Song, S.J., Ma, E.B., Guo, Y.P., 2011. Chronic accumulation of cadmium and its effects on antioxidant enzymes and malondialdehyde in Oxya chinensis (Orthoptera:Acridoidea). Ecotox. Environ. Safe. 74, 1355-1362. [CrossRef]
- Corpas, F.J., González-Gordo, S., Palma, J.M., 2022. NO source in higher plants: present and future of an unresolved question. Trends Plant Sci. 27(2):116-119. [CrossRef]
- Caro, A., Puntarulo, S., 1998. Nitric oxide decreases superoxide anion generation by microsomes from soybean embryonic axes. Physiol. Plantarum.104, 357-364. [CrossRef]
- Huang, A.X., She, X.P., Huang, C., Song, T.S., 2007. The dynamic distribution of NO and NADPH-diaphorase activity during IBA-induced adventitious root formation. Physiol. Plantarum, 130, 240-249. [CrossRef]
- Wang, C. R., Wang, X. R., Tian, Y., Yu, H. X., Gu, X. Y., Du, W. C., & Zhou, H., 2008. Oxidative stress, defense response, and early biomarkers for lead-contaminated soil in Vicia faba seedlings. Environ. Toxicol. Chem. 27(4), 970-977. [CrossRef]
- Yin, Y., Wang, X., Sun, Y., Guo, H., Yin, D., 2008. Bioaccumulation and oxidative stress in submerged macrophyte Ceratophyllum demersum L. upon exposure to pyrene, Environ. Toxicol. 23(3), 328-336. [CrossRef]
Figure 1.
Levels of the superoxide anion O2•- in rice leaves after MC-LR exposure. Data are denoted as mean ± standard deviation (n = 4). Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 1.
Levels of the superoxide anion O2•- in rice leaves after MC-LR exposure. Data are denoted as mean ± standard deviation (n = 4). Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 2.
Levels of GSH in the leaves of rice seedling exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 2.
Levels of GSH in the leaves of rice seedling exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 3.
Level of MDA in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 3.
Level of MDA in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 4.
Activity of SS in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 4.
Activity of SS in the leaves of rice exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 5.
Activity of iNOS (a) and TNOS (b) in the leaves of rice seedlings exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.
Figure 5.
Activity of iNOS (a) and TNOS (b) in the leaves of rice seedlings exposed to MC-LR. Data are denoted as mean ± standard deviation (n = 4). Asterisk indicates statistically significant difference between means of various treatments and control group at each time point.

Figure 6.
Correlation between physiological and biochemical indexes in rice. The size of the circle and the color depth in the Correlation Heat Map represent the size of the Pearson Correlation Coefficient, Asterisk indicates the correlation is significant (p ≤ 0.05). Env-MC: MC-LR concentrations in the nutrient solution; Accum-L: MC-LR contents in rice leaves; Accum-R: MC-LR contents in rice roots.
Figure 6.
Correlation between physiological and biochemical indexes in rice. The size of the circle and the color depth in the Correlation Heat Map represent the size of the Pearson Correlation Coefficient, Asterisk indicates the correlation is significant (p ≤ 0.05). Env-MC: MC-LR concentrations in the nutrient solution; Accum-L: MC-LR contents in rice leaves; Accum-R: MC-LR contents in rice roots.

Table 1.
Bioaccumulation of MC-LR in rice leaves and roots.
| MC-LR concentration, μg/L | Bioaccumulation of MC-LR in rice leaves and roots | ||||||||||
| Leaves, μg/g FW | Roots, μg/g FW | ||||||||||
| Exposure period a, d | BCF b | Exposure period, d | BCF b | ||||||||
| 15 | 20 | 34 | 7 | 15 | 20 | 34 | |||||
| 0.1 | 0.54 ± 0.09 | 0.59 ± 0.08 | 0.51 ± 0.09 | 5.90 ± 0.80 | 0.68 ± 0.05 | 0.77 ± 0.04 | 0.75 ± 0.14 | 0.67 ± 0.05 | 7.70 ± 0.40 | ||
| 1.0 | 0.67 ± 0.06 | 0.69 ± 0.04 | 0.44 ± 0.10 | 0.69 ± 0.042 | 0.68 ± 0.05 | 0.72 ± 0.05 | 0.75 ± 0.03 | 0.71 ± 0.19 | 0.75 ± 0.03 | ||
| 10.0 | 0.85 ± 0.17 | 0.88 ± 0.11 | 0.78 ± 0.06 | 0.088 ± 0.011 | 1.12 ± 0.28 | 1.16 ± 0.16 | 0.98 ± 0.10 | 0.83 ± 0.19 | 0.12 ± 0.02 | ||
| 50.0 | n.a.c | 0.64 ± 0.46 | 0.33 ± 0.07 | 0.013 ± 0.01 | 1.55 ± 0.52 | 0.58 ± 0.05 | 0.46 ± 0.39 | 0.74 ± 0.21 | 0.03 ± 0.01 | ||
Data are denoted as mean ± standard deviation (n = 3). a. We did not collect enough leaves in 7-day experimental group for determination of MC-LR content; b. Here BCF stands for the maximum ratio of MC-LR in rice leaves or roots to the aqueous MC-LR concentration; c. n.a. = not available.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








