Preprint
Article
The Combined Effect of Lemon Peel Extract and Calcium Chloride on the Physical and Biochemical Quality Parameters of Dessert Banana (Musa acuminata var. Dwarf Cavendish) Fruit
Altmetrics
Downloads
138
Views
44
Comments
0
A peer-reviewed article of this preprint also exists.
Abstract
The dessert banana is a popular fruit worldwide, but its ripening process is greatly accelerated by high temperatures, which eventually leads to an unpleasant taste and the appearance of spots on the skin of the fruit. To slow down the ripening of bananas, expensive strategies are used, which are usually not practical for conventional farmers in less developed countries. In this study, we try to find a less costly alternative. Therefore, the effects of coatings of lemon peel extract (2.5 %, 5 %, and 10 %), calcium chloride (4 %), and glycerol (2 %) on the shelf life and postharvest quality of banana fruit (Cavendish) stored at 20 ± 3 °C and 50 – 70 % relative humidity were investigated. Treatment with a mixture of 2.5 % lemon peel extract and 2 % glycerol resulted in an extension of the shelf life of the dessert banana by up to 6 days and no detectable fungal infestation. The mixture is an effective alternative to extend the shelf life and reduce quality losses in bananas.
Keywords:
Subject: Biology and Life Sciences - Plant Sciences
1. Introduction
Banana is one of the most well-known and frequently consumed fruits worldwide. It is valued for its convenient size, ease of transport, and sweet and savory taste. However, aside from their delicious flavour, bananas are also very nutritious and have numerous health benefits. The Cavendish banana variety is the fifth largest agricultural commodity in the world trade after cereals, cassava, sweet potato, and yams and the fourth most important foodstuff in the world after rice, wheat, and milk [1]. Banana plays an important socioeconomic role in developing countries from tropical and subtropical zones, especially in East, Center, and West African countries, from South East of Asia, Central, and South America, and the Caribbean [2]. Cavendish banana production is currently dominated by Indonesia, with the Philippines in second place. The Philippines produced roughly 7.5 million tonnes of bananas in 2020, compared to Indonesia’s over 11 million tonnes. With 2.3, 1.5, and 1.1 million tonnes of Cavendish bananas produced in 2020, the United Nations Organization for Food and Agriculture reports that Ivory Coast, Ghana, and Cameroon are Africa’s top producers of the variety [3].
Banana is not only a rich source of vitamin A, B, and C, manganese, potassium, and fibers but also it is known that 100 g of banana includes roughly 89 kcal calories, 74 g water, 1.1 g protein, 0.3 g lipid, 21.8 g carbohydrate, 2 g fiber, 1 mg sodium, 385 mg potassium, 8 mg calcium, 30 mg magnesium, 0.4 mg iron, 22 mg phosphorous, 11.7 mg ascorbic acid, 40 µg thiamin, 70 µg riboflavin, 610 µg niacin, 80–600 µg pantothenic acid, 470 µg pyridoxine, and 23 µg folic acid [4]. Banana is a climacteric fruit that ripens quickly after harvesting. Thus, it is a perishable fruit and has a very short lifespan between harvest and the onset of deterioration [5]. It is estimated that post-harvest losses in bananas can differ based on several variables, including the variety of bananas, the storage conditions, and the handling methods. In developing countries, post-harvest loss of bananas reaches 30 % or higher because of the poor storing and handling conditions which are frequently unsatisfactory while post-harvest losses are often lower in developed countries with stronger handling and storage infrastructure, averaging around 5–10 % [3]. Many storage methods have been developed to increase the time and distance between harvest and marketing for commodities. For example, the fruit is usually harvested mature and green for commercial use, and sent chilled to the importing nations. Upon arrival at their destination, the green bananas are held in modified atmospheric rooms at the distribution centres where they are treated with chemicals like ethylene gas before selling [6]. By lowering the metabolic rate, minimizing peel greening, and preventing fruit degradation, several other preservation techniques have been investigated, such as regulated and modified atmospheres [7]. Similarly low oxygen pre-treatment for two days can prevent bananas from ripening during storage and shipping [8]. To preserve the color and texture of post-harvest bananas, chemicals are frequently used to delay ripening [9]. Edible coatings [10] and the combinations of chemical dippings with edible coatings [11] are also use. Small-scale farmers in developing countries rely on the natural ripening of bananas, which might be viewed as unsustainable and uneconomical because these processes are highly expensive for them. Because of inadequate storage and ripening conditions, many ripe bananas are lost or spoiled.
Among all the methods of fruit preservation currently in use, the use of edible coatings is a popular practice that helps preserve the nutrients of food, particularly fruits, and vegetables, and offers long shelf lives [12]. Edible coatings help preserve volatile flavour components, slow down microbial growth, delay dehydration, reduce respiration, improve textural quality, and prolong the shelf life of perishable food products [13]. The antioxidant and antifungal qualities of the coating solution give it the ability to delay ripening and senescence. According to recent studies, fruit seeds and peels may potentially have antioxidant capabilities. Examples include mango seed kernels [14] pomegranate peels [15], wampee peels [16], and grape seeds and peels [17]. In the past ten years, several researchers have suggested that citrus waste might be utilized as a natural source of antioxidants [18]. While using glycerol as a plasticizer, calcium chloride has been widely used as a preservative and firming agent for whole and fresh-cut goods. The use of calcium chloride has also been linked to fruit firmness, stress tolerance, ripening, and senescence [19]. There is little information about the combined effect of lemon peel extract, and calcium chloride on banana fruits. Therefore, the objective of this study was to find an alternative solution to extend the shelf life of bananas by using a combination of lemon peel extract, glycerol, and calcium chloride as an edible coating solution and study their effect on the physicochemical and biochemical qualities of the dessert banana fruits.
2. Materials and Methods
2.1. Chemicals and Reagents
The standards lutein, alpha-carotene, beta-carotene, sucrose, fructose, and glucose were obtained from Sigma-Aldrich; acetone, acetonitrile, n-hexane, ethanol (EtOH), methanol (MeOH), methyl tert-butyl ether (MTBE), calcium chloride (CaCl2), and glycerol were purchased from Merck (Darmstadt, Germany). H2O and acetonitrile were of liquid chromatography grade.
2.2. Plant Materials
Sample Preparation and Storage
The banana fruits were purchased fresh from a local supermarket in Germany. They were in the greenest stage conceivable, as indicated by the greenness level being 1. After being delivered to the laboratory, healthy fruits were selected, disassembled, thoroughly washed with fresh tap water, and given about two hours to dry under ambient temperature. Lemon fruits were acquired from a private plantation in Foumbot, Cameroon, which is located in the western part of the country. After removing all the fruits with physiological and physical abnormalities, such as those that were rotten and immature, the fruits were immersed for around 2 hours in water containing sodium hypochlorite (230 µl.L-1) for disinfection. After that, they were rinsed with fresh tap water and the fruit’s peels were removed using a sharp knife. They were crushed with a conventional grinder after they had completely dried in the shade, producing a lemon peel powder.
2.3. Coatings and Storage Conditions
The different coatings were made of different concentrations of lemon peel extract (0 %; 2.5 %; 5 % and 10%), calcium chloride (4 %), and glycerol (2 %). The pH of the solution was adjusted to pH 5.6 with 0.1 mol.L-1 sodium hydroxide. Bananas were dipped into the prepared coating solution for 3 min and allowed for 1 h to dry. Uncoated bananas as control samples (T0) were immersed in distilled water for the same period. The storage room was equipped with LED (light-emitting diodes, manufactured by Sylvania Luxine Plus, Erlangen, Germany) light simulating daylight light (F30W/ 865- T8). The temperature and relative humidity were controlled throughout the storage period. The temperature ranged from 19–22 °C and the relative humidity was recorded with an Efento sensor (Krakow, Poland) from 40 to 60 % and was controlled with an air humidifier (PHILIPS, Amsterdam, Netherlands). The coated and uncoated bananas were stored for a period of 13 d and another 7 d for observation, and quality parameters were recorded at 4 d intervals.
2.4. Experimental Design
Nine edible coating solutions were prepared, and the chosen concentrations were based on previous experiments; T0 = control sample with only distilled water, T1 = distilled water + glycerol (2 %), T2 = glycerol (2 %) + 2.5 % lemon peel extract, T3 = glycerol (2 %) and 5 % lemon peel extract, T4 = glycerol (2 %) + 10% lemon peel extract, T5 = 4% calcium chloride (CaCl2), T6 = glycerol (2 %) + CaCl2 (4 %), T7 = glycerol (2 %), CaCl2 (4 %) and 2.5 % lemon peel extract, T8 = glycerol (2 %), CaCl2 (4 %) and 5 % lemon peel extract, T9 = glycerol (2 %), CaCl2 (4 %), and 10% lemon peel extract. These treatments were arranged in a completely randomized design (CRD) with three replications. Thirty-six fruits were used per treatment and replicated three (3) times. Ten fruits from each replicate were used for non-destructive analyses while 26 were used for destructive analyses.
2.5. Physiological Weight Loss
The weight of each banana finger was measured using an analytical balance (Sartorius Lab Instruments, GmbH & Co. KG, Göttingen, Germany). The fruit weights were recorded at regular intervals throughout storage, and the cumulative physiological weight was determined using the formula below:
where PWL represents the physiological weight loss in percentage, IM, and FM represent the initial mass and the final mass respectively.
2.6. Firmness
The fruit ‘s texture properties were measured using the texture analyzer (Model TA- XT, Stable Micro System Ltd., Surrey GU7, UK). The data was recorded using the EXPONANT software. The firmness of the fruit´s flesh was measured by punching the sample resulting in a plot of strength versus time. Compression force measurement mode, pre-test speed of 1.5 mm.s-1, test speed of 1.0 mm.s-1, post-test speed of 10 mm.s-1, trigger-auto type 2 kg, and data acquisition speed were the working circumstances used to measure the firmness value. The accessories used were a 5 mm cylindrical probe (P/5) and a heavy platform (HDP/90). Triple readings were taken and recorded at three separate locations on the fruit while resting on the texture analyzer’s sturdy platform. The average data in Newton were used.
2.7. Color Analysis
A D90 digital Nikon camera and a 35 mm F/2 D Nikon prime lens were used for colour analysis. Digital photos were captured under controlled LED illumination using a DigiEye imaging system (VeriVide, UK) with the help of DigiEye software version 2.8.0.3. The VeriVide D65 fluorescent tubes are located in the illumination box, which is a lighting cabinet with light having similar characteristics to natural daylight. Before any measurement, the system was calibrated using a white uniform board Digitizer Calibration Pack (version number 4.0) from the Digieye service Pack. Nearly five banana fruits per replicate meaning 15 banana fruits per treatment were placed on the surface of an additional rectangular blue plate in the box. The surface of the plate containing the fruits was illuminated by the mirrors. The blue background of the collected images was removed and the fruit colors were analysed with ImageJ version 1.53K (http://imagej.nih.gov/ij) and the results were obtained in RBG (Red-Blue-Green) value and then converted into La*b* values with Python. These La*b* values determined the total colour difference using the formula below.
where: ΔL represents the change in lightness. L2* and L1* are the lightness values of the two compared samples. a2 and a1 are the red-green colour values of the two compared samples. b2 and b1 are the yellow-blue colour values of the two compared samples. The notation (+ = lighter; − = darker), (+ = redder, − = greener), (+ = yellow, − = bluer) explains the interpretation of the positive and negative changes in lightness (ΔL), red-green (Δa), and yellow-blue (Δb) colour values.
△ E = [(△L) 2 + (△ a *) 2 + (△ b *) 2] ½
ΔE = [ (L2 − L1)2 + (a2 − a1)2 + (b2 − b1)2] ½
2.8. Decay Percentage
The percentage of decay or rot was determined by visual observation. The survey excluded the rotten fruits caused by physiological and/or microbiological disorders, and the percentage was estimated.
2.9. Total Soluble sugar, Titratable Acidity, and pH
A 5 g sample of the banana pulp was prepared from 3 fruit and homogenized with ultra-turrax and 15 mL of MQ water. The plant material was centrifuged for 10 min at 3,000 rpm. Total soluble sugar was determined by a digital refractometer (Atago, Pocket Brix-Acidity Meter, Tokyo 105-0011, Japan). A volume of 45 mL of MQ water was used to dilute and homogenize 5 mL of the fruit juice. The pH was measured with a digital pH meter (EC-30-PH PHOENIX instrument from ProfiLab24 GmbH, Berlin, Germany). By titrating the diluted fruit juice using a magnetic stirrer in the presence of phenolphthalein, the titratable acidity was determined and calculated using the following formula:
Titratable Acidity (TA) = (Volume of NaOH (in mL) × 0.1 (normality of NaOH) × 0.064) / (Total juice volume in mL) × 100. Where the volume of NaOH (in mL) is the volume of sodium hydroxide solution (in milliliters) used to neutralize the acidity in the juice sample during the titration process; 0.1 is the normality of the sodium hydroxide (NaOH) solution and represents its concentration; 0.064 is the citric acid milliequivalent factor and total juice volume (in mL) is the volume of the juice sample used in the titration (before the addition of NaOH).
2.10. Sugar Analysis
Ten grams of the banana pulp of each treatment were ground, diluted, and centrifuged at 13000 rpm for 10 min. The supernatant was collected, dried by lyophilization, and kept at -4 °C for further analysis. 3The used HPLC system was equipped with an S5200 autosampler and a pump 1000 Smartline with Manager 5000 (Göbel Instrumentelle Analytik GmbH, Hallertau, Germany). The detector was an evaporative light scattering detector (ELSD; PL-ELS 2100, Polymer Laboratories GmbH, Darmstadt, Germany). The column (Grace GmbH, Germany) was Allsphere Amino 250x4.6 mm, 5 µm. MilliQ water (ultrapure water) was utilized as eluent A (20 %) and acetonitrile as eluent B (80 %). The flow rate was 1.0 mLmin-1 at 30 °C. The parameters for the ELSD were Evap:80, Neb:45, Gas:1.6, Pmt:6, Smth:1, and LED:100. The data management system was Geminix III software, version 1.10.3.9 (Göbel Instrumentelle Analytik). Sucrose, glucose, and fructose standards were used for identification and quantification.
2.11. Carotenoids Analysis
Ten grams of banana pulp were extracted using ethanol/n-hexane (4:3 v/v). After centrifugation at 13,500 rpm for 10 min., the yellow supernatant was collected and re-extracted with the same solvent until it had become colorless. The supernatants were combined and dried under reduced pressure with a rotatory evaporator. The residues were dissolved with n-hexane and redried. One hundred µL of ethyl acetate was added to the dried samples, vortexed, and centrifuged at 10,000 rpm. The analysis was carried out on the Agilent Technologies 1260 Infinity HPLC (Agilent, Waldbronn, Germany) equipped with a column size of 250x4.6 mm (RP-18; YMC CO., LTD). The eluents were methanol (eluent A: 20 %) and tert.-butyl methyl ether (eluent B: 80 %). Carotenoids were detected at 440 nm. The injection volume was10 µL and the flowrate was 1 mL.min-1. The signals were converted and recorded by OpenLab software version 2.4. Identification and quantification of individual carotenoids were carried out by comparing retention times and UV/Vis absorption with those of authentic standards.
2.12. Statistical Analysis
With treatment and storage time as sources of variation, all quantitative parameter data were subjected to a one-way ANOVA using R version 4.1.2 (2021-11-01) statistical analysis software with its cross-platform development environment called Rstudio. Data were expressed as mean plus standard deviation (SD) from the three replicates. Turkey tests, compared at p < 0.05, were used to establish the level of significance of the difference between the different treatments.
3. Results
3.1. Texture Analysis
The changes in texture or firmness of the banana fruit with and without coating during the storage period are shown in Table 1. All treatments showed comparable firmness values (p > 0.05) after one day of storage. Significant firmness changes between treatments were observed after 9 days of storage (P < 0.05). In comparison to other treatments, T2 had the highest firmness value (32.1 ± 3.2 N), indicating improved firmness preservation. The lowest value measured (2.6 ± 0.3) was observed for T9, showing a considerable loss of firmness during storage. After 13 days, there were still differences between the treatments in the firmness values, which ranged from 2.3 N to 9.6 N. However, there were no significant variations between the treatments (p > 0.05). The firmness of fruits is crucial in determining how resistant they are to mechanical injury. The different treatments of the samples affected how well the firmness was preserved during storage. The results thus demonstrate the impact of the concentration of lemon peel extract (LPE) on the firmness of bananas during storage. Bananas treated with CaCl2 alone (T5) were also firmer than untreated fruit (T0) after, 9 and 13 days of storage. This result is in line with the recent observation who reported that banana fruits treated with CaCl2 have a slower rate of deterioration and texture loss [20]. In addition, CaCl2 treatment has been proven to retain effectively the firmness of chili pepper [21]. However, others reported that CaCl2 solution accelerates the ripening process of bananas [22]. In fact, fruit softening and textural changes are brought on by the depolymerization and solubilization of cell wall constituents as well as cell structure degradation [23].
3.2. Physiological Weight Loss
Due to physiological changes and water loss, banana fruits lose weight during storage. Therefore, the influence of edible coatings on the physiological weight loss (PWL) of banana fruits during storage was studied (Figure 1). As the storage time increased, the physiological weight loss in all the treatments also increased as a result of the normal ripening process in fruits. On the first day (day 1) after coating, no (0 %) physiological weight loss was recorded in both coated and non-coated banana fruits, meaning that the application of the edible coating formulation has no immediate effect on the water content of the banana. The physiological weight loss values varied at day 5 from 2.4 % to 6.2 % where some treatments exhibited noticeable differences in PWL compared to the control T0. T1 and T9 showed higher PWL values of 6.1 % and 6.4 %, respectively, indicating that the coating might have limited effectiveness in reducing PWL at this time point, but T2 demonstrated a high reduction in PWL with a value of 4.9 %, suggesting that the coating formulation in T2 has a positive impact on preserving banana weight during this period. On day 9, all treatments displayed a noticeable reduction in PWL compared to the control T0. T2, T3, and T6 showed a high reduction in PWL with values of 5.2 %, 7.6 %, and 4.9 %, respectively. The results indicate that the effect of the edible coating becomes more evident with increasing storage time. On day 13, when all the fruits were ripe, a high value of PWL was observed in all treatments in comparison to the control T0 (2.3 %) which was already at the senescent phase. T2 continued to exhibit the highest reduction in PWL with 4.9 %. Usually, the main cause of physiological weight loss for fruit and vegetables is transpiration, which is dictated by the difference in water vapour pressure between the fruit and the atmosphere [24]. Furthermore, edible coatings act as a semipermeable barrier against oxygen, carbon dioxide, moisture, and solute movement, so lowering respiration, water loss, and oxidation reaction rate [25].
3.3. Decay Percentage
The bananas that showed decay after day 13 were counted and expressed as a percentage (Figure 2). T2 showed the lowest number of damaged bananas with a decay percentage of 2.3 % ± 0.6 %, compared to the other treatments. A low concentration of LPE (2.5 %) had a strong positive effect on the shelf life of the bananas, while a high concentration of LPE (5 and 10 %) accelerated the deterioration of the fruits e.g T3 (34.4 % ± 1.5 %) and T4 (31.3 % ± 1.5 %), respectively. Similarly, CaCl2 treatment (T5) reduced the decay of banana in comparison with the control (T0) as only 11.0 % ± 1 % of the fruits were spoiled compared to T6 (31.6 % ± 2.0 %), T7 (28 % ± 2 %), T8(19.3 % ± 2.5 %), and T9 (23.6 % ± 2.0 %). The reduction of the decay of the fruits after the application of LPE as an edible coating on the surface of fruits could be explained by the antifungal effect of lemon peel. The effect of the essential oil of lemon, mandarin, grapefruit, and orange on the growth of moulds commonly associated with food spoilage has already been shown [26].
Bananas were coated differently (see Material and Methods, experimental design) and the fruits that went bad after storage were counted and expressed as a percentage. Different letters mean that the values are significantly different (p < 0.05).
3.4. Total Soluble Sugar
The total amount of soluble sugars present in a liquid or solution is referred to as total soluble sugars, also known as the total soluble solids (TSS). The total soluble sugar (TSS, °Brix) of the coated and uncoated bananas was determined during the storage (Table 1). All treatments had TSS values between 2.1 and 2.1 % Brix after an initial storage period of 1 day. The TSS values significantly differed between the treatments after the 5-day storage period. In comparison to other treatments, T0, T1, T4, T8, and T9 exhibited higher TSS values (ranging from 3.0 to 3.4 % Brix), indicating a faster ripening of these bananas in comparison with the fruits treated differently. The TSS readings continued to deviate when the storage duration increased. TSS values for T0, T3, and T4 showed the highest values with 16.8 ± 0.2, 16.6 ± 0.1, and 16.5 ± 0.1 % Brix, respectively at day 9. Similarly, results differed significantly at day 13. TSS values for T0 were highest at 17.8 ± 0.1 % Brix, indicating that all other treatments slowed the ripening process. These results of the effect of edible coatings are similar to those obtained on strawberries [27] and bananas [28]. The treatment T2 was found to be the most effective in retaining TSS during the storage period. The effect of CaCl2 was not significant enough to maintain TSS compared to other edible coating formulations. Coating film on the surface of the banana reduces the internal respiration rate and vital processes, which reduces the ripening process and keeps the TSS value low.
3.5. Color Analysis
The La*b* colour space offers insightful information about the samples’ colour properties. In this study, the change in La*b* values over time for various treatments in comparison to the control (T0) was studied (Table 2). The value L* stands for the lightness component, whereas a* and b* stand, respectively, for the red-green and yellow-blue colour components. At day 1, all treatments showed comparable L* values in comparison to the control (T0), showing that there were no appreciable variations in the samples’ lightness. The a* and b* values were also similar to T0, indicating that there were no significant differences in the red-green and yellow-blue colour components. Day 5 analysis showed that T1 had a considerably higher a* value than T0 (p < 0.05). T1 showed a shift towards a more intense red or green hue compared to T0 with an average a* value of 91.6 ± 0.5. The L and b* values between treatments did not show any discernible variations at this time. On day 9, when compared to T0, T2 had significantly lower L levels (p < 0.05). T2 had a darker appearance than T0, with an average L value of 90.3 ± 1.3. Furthermore, b values in T2 were considerably lower than in T0 (p < 0.05) and showed a trend towards a more vivid blue or yellow colour with an average b value of 12.4 ± 0.1. The values between treatments did not show any discernible variations at this period. However, when compared to the other treatments, T6 displayed a considerably higher b value at day 9 (7.5 ± 0.1). This suggests that the yellow colour of T6 was more pronounced. However, treatment T2 was greener than the other treatments on day 13. This can be concluded by the lower a* value of T2 (6.5 ± 4.5) on day 13 in comparison to the other treatments. The CIELAB colour space’s green-red axis is represented by the* value, where negative values denote greener hues. As a result, T2 had a greener appearance on day 13 than the other treatments. The results show that T3, T4, T9, and T2 retain effectively the most chlorophyll content (green colour) of the fruit while the other coated fruits are yellow due to the carotenoid content of the fruit´s peel. The green hue of the banana peel gradually fades during ripening as a result of the thylakoid membrane breaking and the chlorophyll being degraded by chlorophyllase and oxidase enzymes, revealing yellow carotenoid pigments [29]. LPE could retain effectively the green colour of banana fruit, while the combination of LPE and CaCl2 could reduce this effect. This is well observed in T8 where we have a 5 % concentration of LPE in combination with CaCl2 (4 %), which has less effect on the retention of green colour during the storage time as compared to T3 with 5 % LPE concentration without CaCl2 which presents a stronger effect on the green colour retention. However, during storage, L values fell for both coated and uncoated samples, possibly as a result of surface moisture loss, which could have resulted in the darker hue seen [30]. Similar results were obtained in the study of an edible coating based on quince seed gum at various concentrations on banana slices at 4 °C and 40 °C [31].
Coating formulations. Coating film on the surface of the banana reduces the internal respiration rate and vital processes, which reduces the ripening process and keeps the TSS value low.
3.6. Titratable Acidity, and pH
The changes in the pH of control and treated banana fruits during the storage period were determined (Figure 3). The pH increased as the storage time increased. On day 1, there is no difference between the pH of the coated and the control fruits whose value varies between 3.7 and 3.8. By day 9 and 13, the pH values increased uniformly and T2 showed the lowest pH throughout the storage period with values of 4.3 and 4.8 on day 9 and 13 respectively, while the control sample had values of 5.2 and 6.6 on days 9 and 13 respectively. No significant differences were found in the other formulations compared to the control samples.
Titratable acidity (TA) is a measure of the acidity of a solution, expressed in terms of the amount of a strong base needed to neutralize the acid in the solution. Citric acid is one of the major organic acids in ripe banana fruits. The TA gradually increased as the storage time increased (Figure 4). After the first week of storage, the TA was not significantly different between the coated and non-coated fruits. By day 13, the values of T4, T5, T6, T7, T8, and T9 were higher than the control while T3 and T2 were lower. This result showed that formulation T2 causes the titratable acidity to remain low as in the control on day 0. The coating T2 might reduce the metabolic activity and lower the fruit respiration of banana fruit. These results are in accordance with the results obtained on bananas coated with chitosan and with chitosan in combination with gibberellic acid [25].
3.7. HPLC Sugar Analysis
Glucose, fructose, and sucrose are the main sugars in banana fruit during ripening and these sugars increase in concentration during ripening [32]. The concentration of these sugars in coated and uncoated bananas was determined during the storage period (Figure 5). In general, the sugar content increased as the storage time increased. The concentrations were not significantly different from each other up to the first 9 days of storage. By day 9, the highest sucrose concentration was in T0 (2.1 mg.g-1), T6 (2.0 mg.g-1), and T7 (1.8 mg.g-1) while the lowest was recorded in T8 (0.8) mg.g-1), and T2 (1.3 mg.g-1). The sucrose concentration of T0 dropped to 0.7 mg.g-1 at day 13 while in all the coated samples the sucrose content increased. T1, T2, and T5 showed the highest sucrose concentrations at day 13 with 2.3 mg.g-1, 2.2 mg.g-1, and 2.2 mg.g-1, respectively. The fructose concentrations were higher in most of the coated fruit as compared to the control samples. By day 9, the concentration of fructose and glucose were highest in T4 (1.2 mg.g-1; 1.2 mg.g-1), T7 (1.8 mg.g-1; 1.1 mg.g-1), T6 (1.1 mg.g-1; 1.1 mg.g-1) and T3 (1.1 mg.g-1; 1.1 mg.g-1), while the lowest content was observed in T2 (0.7 mg.g-1; 0.7 mg.g-1), and T0 had values of 0.8 mg.g-1; 0.8 mg.g-1 for fructose and glucose, respectively. T0 fructose and glucose concentrations dropped to 0.7 mg.g-1 and 0.7 mg.g-1 on day 13 while the concentration of sugars in the coated fruits continued to increase. But among the coated fruits T8 (0.9 mg.g-1; 1.1 mg.g-1), T1 (0.8 mg.g-1; 0,8 mg.g-1), and T2 (0.8 mg.g-1; 0.8 mg.g-1) contained low fructose-glucose content. A complicated regulatory process changes metabolism during banana ripening from starch synthesis to starch breakdown, resulting in the accumulation of soluble sugars, primarily sucrose, which significantly affects the taste and flavour of the fruit. This conversion of starch into sucrose appears to be responsible for sweetening the pulp and providing energy for metabolic processes that lead to the development of other quality characteristics of ripe bananas, such as colour change, synthesis of volatile compounds, and even softening of the pulp, which greatly affects the quality of the finished fruit [33]. In all the samples a high concentration of sucrose compared to the concentration of fructose and glucose was detected. In fact, during the ripening process, glucose and fructose are formed from sucrose molecules. When the climacteric peak is reached, this sucrose concentration starts decreasing under the effect of saccharolytic enzymes, while fructose and glucose concentration increase. Several authors have tracked the concentration of starch, sucrose, glucose, and fructose as well as the activities of various enzymes involved in the synthesis of sucrose [34]. In the control sample T0, a typical natural ripening is observed (Figure 5), as evidenced by degradation of sucrose at day 13, while all coated samples still show a high sucrose content at day 13. From an organoleptic point of view, the coated fruits could be sweeter, since the total content of sweet-tasting sugars is higher in the coated fruit samples, which is an important criterion for consumer acceptance.
3.8. HPLC Carotenoids Analysis
The distinctive yellow colour of ripe banana peel is solely the result of chlorophyll breakdown, which obscures the yellow hue of unripe bananas. As a result, the carotenoid content in banana changes as the fruit ripening and maturation proceeds. The concentration of carotenoids (lutein, alpha-, and beta-carotene) was analysed in the coated and non-coated bananas during the storage period (Figure 6). The concentration of carotenoids increased with increasing storage time. Lutein was the major carotenoid, followed by alpha-, and beta-carotene. A large difference in carotenoid concentration was observed particularly at day 5 of the storage time, when all the treated banana fruits had a higher lutein concentration compared to the control samples that had a concentration of 0.2 mg.g-1. The highest concentration was detected in T1 (0.3 mg.g-1), T2 (0.38 mg.g-1), T7 (0.37 mg.g-1) and T9 (0.30 mg.g-1) while the alpha- and beta- carotene content remained low. At day 9 alpha-, and beta-carotene concentration were particularly high in T2 with 0.14 mg.g-1 and 0.05 mg.g-1, respectively. In the control, beta-carotene was not detected at day 9. The highest concentration of lutein was found in the control sample T0 with 0.5 mg.g-1 at day 13. Fruit like banana produces a large amount of carotenoids, which are produced from terpenoids, as the chloroplast to chromoplast transition occurs [35]. During the ripening process, the fruit colour changes due to the production of carotenoids and the destruction of chlorophylls. As in regular ripening, the highest level of lutein content in T0 and T5 compared to the other coated samples at day 13. This could indicates that T0 and T5 are in a more advanced stage of the ripening process. Thus, coating delays the ripening of the banana.
4. Conclusions
The present results lead to the conclusion that banana fruits coated post-harvest with lemon peel extract-based formulations retain significant fruit chlorophyll content despite a reduction in fruit firmness.. The addition of CaCl2 in the formulation reduced the effect of the lemon peel extract but was not enough to be considered important. The use of a concentration of 2.5 % lemon peel extract in the formulation of an edible coating for the post-harvest preservation of bananas showed a significant retention of color, sugar, firmness and carotenoids of the fruit, which are very important parameters for quality control, as a look at the behavior of treatment T2 clearly shows throughout the storage period. On the other hand, a high percentage of lemon peel extract (here 10 % in T3) in the edible coating solution applied to the surface of bananas causes the fruit to ripen very quickly compared to the control samples. These data provide significant and helpful information for keeping the quality of bananas in the fresh produce post-harvest management. The findings of this research greatly contribute to the income improvement for farmers, processors, and distributors to market banana fruit on trade in developing and emerging countries.
Author Contributions
Eric-Ivan Ngoko Tchamba: Conceptualization, investigation, methodology, analysis, writing—original draft, writing review & editing. Wilfried Schwab: Supervision, conceptualization, investigation, methodology, analysis, resources, writing—review & editing, validation. Francois Nguetsop: Supervision, methodology, Project administration, visualization. Jean Aghofack-Nguemezi: Supervision, Project administration, conceptualization, investigation, methodology. Peter Muranuyi: conceptualization, methodology, analysis. Thorsten Tybusseck: conceptualization, methodology, Formal analysis, Software, Data curation.
Funding
This work was supported by the DAAD (Deutscher Akademischer Austauschdienst) and by the Professorship Biotechnology of Natural Products.
Acknowledgments
Thomas Hoffmann, Anja Forstner, Mechthild Mayershofer, and Sadiq Mareai are acknowledged for their technical support.
Conflicts of Interest
None.
Abbreviations
| LPE | Lemon peel extract |
References
- Picq, V.; Ramillon, J.; Balanzat, E. Swift heavy ions on polymers: Hydrocarbon gas release. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 1998, 146, 496–503. [Google Scholar] [CrossRef]
- Karamura, D.; Frison, E.; Karamura, D.A.; Sharrock, S. Banana production systems in eastern and southern Africa. Banan. Food Secur. 1998, 401–412. [Google Scholar]
- In Brief to The State of Food Security and Nutrition in the World 2023; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023. [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Dwivany, F.M.; Aprilyandi, A.N.; Suendo, V.; Sukriandi, N. Carrageenan Edible Coating Application Prolongs Cavendish Banana Shelf Life. Int. J. Food Sci. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Palta, J.P. A Postharvest Dip Treatment with of Banana Fruit. HortScience 2015, 50, 1035–1040. [Google Scholar] [CrossRef]
- Song, J.; Fan, L.; Forney, C.; Campbell-Palmer, L.; Fillmore, S. Effect of hexanal vapor to control postharvest decay and extend shelf-life of highbush blueberry fruit during controlled atmosphere storage. Can. J. Plant Sci. 2010, 90, 359–366. [Google Scholar] [CrossRef]
- Imahori, Y.; Yamamoto, K.; Tanaka, H.; Bai, J. Residual effects of low oxygen storage of mature green fruit on ripening processes and ester biosynthesis during ripening in bananas. Postharvest Biol. Technol. 2013, 77, 19–27. [Google Scholar] [CrossRef]
- Silva, V.; Arquelau, P.; Silva, M.; Augusti, R.; Melo, J.; Fante, C. Use of Paper Spray-Mass Spectrometry to Determine the Chemical Profile of Ripe Banana Peel Flour and Evaluation of Its Physicochemical and Antioxidant Properties. Quimica Nova 2020, 43, 579–585. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Fuchs, C.; Yeh, S.-Y.; Huang, F.-C.; Hoffmann, T.; Schwab, W. An oxygenase inhibitor study in Solanum lycopersicum combined with metabolite profiling analysis revealed a potent peroxygenase inactivator. J. Exp. Bot. 2010, 62, 1313–1323. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Hoffmann, T.; Schwab, W. Effects of bio-based coatings on the ripening and quality attributes of tomato (Solanum lycopersicum) fruits. J. Sci. Food Agric. 2018, 99, 1842–1849. [Google Scholar] [CrossRef]
- Ghasemzadeh, R.; Karbassi, A.; Ghoddousi, H.B. Application of Edible Coating for Improvement of Quality and Shelf-life of Raisins. World Appl. Sci. J. 2008, 3, 82–87. [Google Scholar]
- Taylor, P. Edible Films and Coatings: Tomorrow’s Packagings: A Review Edible Films and Coatings: Tomorrow ’ s Packagings: A Review. 2010, 37–41. [Google Scholar]
- Kabuki, T.; Nakajima, H.; Arai, M.; Ueda, S.; Kuwabara, Y.; Dosako, S. Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem. 2000, 71, 61–66. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Singh, R.P. Studies on Antioxidant Activity of Pomegranate (Punica granatum) Peel Extract Using in Vivo Models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Xie, H.; Hao, J.; Yang, B.; Qiu, S.; Wei, X.; Chen, F.; Jiang, Y. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2010, 118, 62–66. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2002, 87, 41–44. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N.; Grohmann, K. Biological Properties of Citrus Flavonoids Pertaining to Cancer and Inflammation. Curr. Med. Chem. 2001, 8, 135–153. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11172671. [CrossRef] [PubMed]
- Martín-Diana, A.; Rico, D.; Frías, J.; Barat, J.; Henehan, G.; Barry-Ryan, C. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 210–218. [Google Scholar] [CrossRef]
- Minh, N.P. Effectiveness of CaCl2 treatment on quality attributes of banana fruit during storage. Plant Sci. Today 2021, 9, 206–214. [Google Scholar] [CrossRef]
- Tunde-Akintunde, T. Effect of Pretreatment on Drying Time and Quality of Chilli Pepper. J. Food Process. Preserv. 2010, 34, 595–608. [Google Scholar] [CrossRef]
- Wills, R.; Tirmazi, S.; Scott, K. Effect of postharvest application of calcium on ripening rates of pears and bananas. J. Hortic. Sci. 1982, 57, 431–435. [Google Scholar] [CrossRef]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced Ripening Agents and Their Effect on Fruit Quality of Banana. Int. J. Food Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.; Jamir, A.; Ankalagi, N.; Henny, T.; Devi, O. Edible coatings in fruits and vegetables: A brief review. Pharma Innov. J. 2021, 10, 71–78. Available online: https://www.researchgate.net/profile/Manisha-Ch- Momin/publication/352994139_Edible_coatings_in_fruits_and_vegetables_A_brief_review/links/60e2f066 299bf1ea9ee13a3d/Edible-coatings-in-fruits-and-vegetables-A-brief-review.pdf.
- Gol, N.B.; Rao, T.V.R. Banana Fruit Ripening as Influenced by Edible Coatings. Int. J. Fruit Sci. 2011, 11, 119–135. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Amal, S.H.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving Strawberry Fruit Storability by Edible Coating as a Carrier of Thymol or Calcium Chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. Available online: www.idosi.org/jhsop/2(3)10/2.pdf.
- Rahman, M.; Hossain, T.; Hossain, M.; Sattar, S.; Das, P.C. Effect of banana peel extract on storage stability of banana cv. Sagar. Food Res. 2019, 4, 488–494. [Google Scholar] [CrossRef]
- Özdemir, I.S. Effect of light treatment on the ripening of banana fruit during postharvest handling. Fruits 2016, 71, 115–122. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Amraie, M.; Salehi, M. Color and weight changes of fresh-cut banana slices coated by quince seed gum: Effect of concentration, storage temperature and duration. Iran. Food Sci. Technol. Res. J. 2019, 14, 75–85. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Cordenunsi-Lysenko, B.R.; Nascimento, J.R.O.; Castro-Alves, V.C.; Purgatto, E.; Fabi, J.P.; Peroni-Okyta, F.H.G. The Starch Is (Not) Just Another Brick in the Wall: The Primary Metabolism of Sugars During Banana Ripening. Front. Plant Sci. 2019, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Wathelet, B.; Paquot, M.; Emaga, T.H. Changements texturaux et biochimiques des fruits du bananier au cours de la maturation. Leur influence sur la préservation de la qualité du fruit et la maîtrise de la maturation. Biotechnol. Agron. Soc. Environ. 2008, 12, 1335–1342. [Google Scholar]
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. Open Archive TOULOUSE Archive Ouverte (OATAO) Mechanism of Fruit Ripening. Dev. Biol. 2010, 1. [Google Scholar]
Figure 1.
Influence of different edible coating formulations on the physiological weight loss of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 1.
Influence of different edible coating formulations on the physiological weight loss of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 2.
Decay percentage of coated and uncoated bananas on day 13.
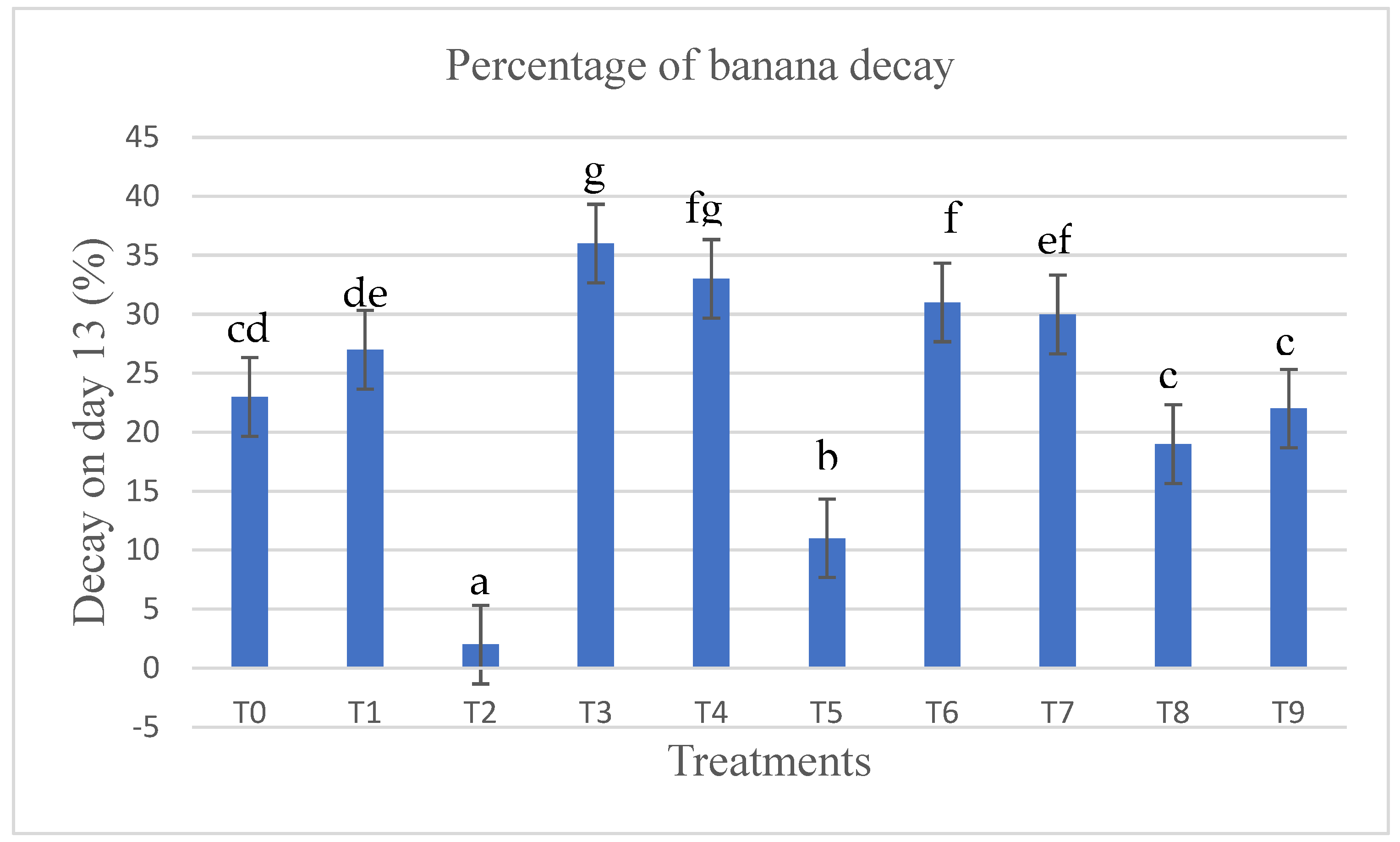
Figure 3.
Influence of different edible coating formulations on the pH of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 3.
Influence of different edible coating formulations on the pH of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 4.
Influence of different edible coating formulations on the titratable acidity of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 4.
Influence of different edible coating formulations on the titratable acidity of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 5.
Concentration of sugars in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 5.
Concentration of sugars in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 6.
Concentration of carotenoids in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 6.
Concentration of carotenoids in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Table 1.
Effect of edible coating formulations on the firmness, physiological weight loss (pwl), and total soluble sugar (TSS) of banana during different storage times at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Table 1.
Effect of edible coating formulations on the firmness, physiological weight loss (pwl), and total soluble sugar (TSS) of banana during different storage times at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
| Storage period (days) | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| 1 | 38.8 ± 0.1 a | 38.2 ± 0.1 a | 38.6 ± 0.5 a | 38.5 ± 0.4 a | 38.8 ± 0.1 a | 38.5 ± 0.1 a | 38.3 ± 0.1 a | 38.5 ± 0.1 a | 38.5 ± 0.1 a | 38.3 ± 0.6 a | |
| Firmness (N) | 5 | 32.3 ± 0.8 a | 33.4 ± 1.5 a | 37.3 ± 2.4 a | 34.4 ± 3.3 a | 32.2 ± 2.2 a | 34.7 ± 2.8 a | 34.4 ± 1.7 a | 33.5 ± 0.6 a | 33.1 ± 1.3 a | 33.7 ± 3.3 a |
| 9 | 2.9 ± 0.1 a | 3.5 ± 0.1 a | 32.1 ± 3.2 b | 3.1 ± 0.4 a | 3.4 ± 0.5 a | 5.3 ± 1.4 a | 5.4 ± 2.4 a | 6.4 ± 4.9 a | 15.3 ± 19.4 ab | 2.6 ± 0.3 a | |
| 13 | 2.3 ± 0.4 a | 2.8 ± 0.3 a | 9.6 ± 1.0 a | 2.4 ± 0.4 a | 2.3 ± 0.1 a | 2.9 ± 0.3 a | 2.9 ± 0.2 a | 2.6 ± 0.5 a | 3.4 ± 0.1 a | 2.7 ± 0.4 a | |
| 1 | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | |
| TSS (%) | 5 | 3.0 ± 0.1 d | 3.0 ± 0.1 d | 2.4 ± 0.1 a | 2.7 ± 0.1 b | 3.2 ± 0.1 e | 2.7 ± 0.1 b | 2.9 ± 0.1 c | 2.7 ± 0.1 b | 3.3 ± 0.1 f | 3.4 ± 0.1 f |
| 9 | 16.8 ± 0.1 i | 13.2 ± 0.1 e | 3.3 ± 0.1 a | 16.6 ± 0.1 h | 16.5 ± 0.1 h | 13.5 ± 0.1 f | 12.6 ± 0.1 d | 11.7 ± 0.1 c | 9.6 ± 0.1 b | 15.3 ± 0.1 g | |
| 13 | 17.8 ± 0.1 f | 16.7 ± 0.2 e | 13.5 ± 0.1 a | 16.6 ± 0.1 de | 15.7 ± 0.1 b | 16.3 ± 0.1 cd | 15.9 ± 0.1 bc | 16.4 ± 0.2 de | 15.7 ± 0.2 b | 16.4 ± 0.2 de |
Note: Mean values followed by the same letter within each parameter (Firmness, PWL, and TSS) are not significantly different at p < 0.05.
Table 2.
Effect of edible coating formulations on the colour of banana storage at 19–22 °C and 40–60 % relative humidity.
Table 2.
Effect of edible coating formulations on the colour of banana storage at 19–22 °C and 40–60 % relative humidity.
| Treatments | Color (CIELab) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||
| L1* | L2* | L3* | L4* | a1* | a2* | a3* | a4* | b1* | b2* | b3* | b4* | |
| T0 | 61.8 ± 0.1 | 91.4 ± 0.9a | 91.4 ± 0.9c | 66.4a ± 0.8a | -9.8 ± 0.1 | -243.6 ± 2.9 | 14.5 ± 0.2c | 12.1 ± 0.3a | 12.0± 0.2 | 14.7 ± 0.7 a | 19.7 ± 0.3 d | 12.4±0.9a |
| T1 | 61.1 ± 0.1 | 91.5 ± 0.5a | 91.5 ± 0.5a | 68.1a ± 0.8a | -5.4 ± 0.1 | -240.27 ± 0.71 | 14.6 ± 0.8a | 13.4 ± 0.7a | 6.2 ± 0.1 | 15.2 ± 0.2 a | 18.6 ± 0.2 ad | 11.4±1.5a |
| T2 | 62.3 ± 0.1 | 90.3 ± 1.2a | 90.3 ± 1.2ab | 65.8a ± 0.4a | -8.0 ± 0.1 | -241.8 ± 1.5 | 15.1 ± 0.6ac | 11.8 ± 0.4a | 12.4 ± 0.1 | 15.4 ± 0.5 a | 17.9 ± 0.2 ab | 12.1±0.3a |
| T3 | 61.8 ± 0.1 | 92.1 ± 0.6a | 92.1 ± 0.6bc | 67.5a ± 1.4a | -7.4 ± 0.1 | -237.9 ± 0.6 | 15.3 ± 0.7ac | 12.7 ± 1.5a | 6.8 ± 0.1 | 15.1 ± 0.3 a | 19.1± 0.4 bd | 11.6±1.5a |
| T4 | 61.7 ± 0.1 | 91.4 ± 0.8a | 91.4 ± 0.8ac | 65.3a ± 0.2a | -6.8 ± 0.1 | -240.5 ± 2.6 | 15.9 ± 0.2ac | 11.8 ± 0.4a | 7.5 ± 0.1 | 15.4 ± 0.3 a | 18.2 ± 0.4 abc | 11.3±0.7a |
| T5 | 61.1 ± 0.1 | 91.8 ± 0.8a | 91.8 ± 0.8ab | 67.5a ± 1.2a | -5.4 ± 0.1 | -239.8 ± 3.5 | 15.6 ± 0.6ab | 12.0 ± 1.5a | 6.2 ± 0.1 | 14.6 ± 0.7 a | 17.7 ± 0.3 a | 12.5±1.5a |
| T6 | 61.70 ± 0.1 | 92.0 ± 0.5a | 92.0 ± 0.5ab | 68.3a ± 1.3a | -6.8 ± 0.1 | -239.5 ± 1.2 | 16.0 ± 0.4ab | 12.2 ± 1.3a | 7.5 ± 0.1 | 15.0 ± 0.2 a | 17.8 ± 0.6 a | 11.8±1.2a |
| T7 | 62.2 ± 0.1 | 92.0± 0.6a | 92.0 ± 0.6ac | 66.5a ± 2.2a | -8.0 ± 0.1 | -239.1 ± 1.9 | 16.0 ± 0.4ac | 11.0 ± 3.0a | 12.4 ± 0.1 | 15.2 ± 0.4 a | 18.8 ± 0.5ad | 10.8±0.8a |
| T8 | 61.8 ± 0.1 | 91.5 ± 0.5a | 91.5 ± 0.5ab | 69.8a ± 4.0a | -9.8 ± 0.1 | -240.4 ± 1.5 | 16.4 ± 1.0a | 13.3 ± 3.0a | 12.2 ± 0.1 | 15.3 ± 0.4 a | 18.0 ± 0.4 ab | 11.6±0.8a |
| T9 | 61.9 ± 0.1 | 91.9 ± 1.2a | 91.9 ± 1.2bc | 66.2a ± 2.4a | -5.2 ± 0.1 | -238.4 ± 2.3 | 17.1 ± 0.4bc | 12.1 ± 1.6a | 6.3 ± 0.1 | 14.6 ± 0.9 a | 19.3 ± 0.6 cd | 11.3±0.5a |
L1, a1*, and b1* represents the L, a*, and b* values on day 1; L2, a2*, and b2* on day 5; L3, a3*, b3* on day 9; L4, a4*, and b4* on day 13. Means with different superscripts are significantly different in their respective column or rows (P < 0.05). storage condition s T0–T9 are described in detail in the Materials and Methods section (experimental design).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Alerts
Abstract
The dessert banana is a popular fruit worldwide, but its ripening process is greatly accelerated by high temperatures, which eventually leads to an unpleasant taste and the appearance of spots on the skin of the fruit. To slow down the ripening of bananas, expensive strategies are used, which are usually not practical for conventional farmers in less developed countries. In this study, we try to find a less costly alternative. Therefore, the effects of coatings of lemon peel extract (2.5 %, 5 %, and 10 %), calcium chloride (4 %), and glycerol (2 %) on the shelf life and postharvest quality of banana fruit (Cavendish) stored at 20 ± 3 °C and 50 – 70 % relative humidity were investigated. Treatment with a mixture of 2.5 % lemon peel extract and 2 % glycerol resulted in an extension of the shelf life of the dessert banana by up to 6 days and no detectable fungal infestation. The mixture is an effective alternative to extend the shelf life and reduce quality losses in bananas.
Keywords:
Subject: Biology and Life Sciences - Plant Sciences
1. Introduction
Banana is one of the most well-known and frequently consumed fruits worldwide. It is valued for its convenient size, ease of transport, and sweet and savory taste. However, aside from their delicious flavour, bananas are also very nutritious and have numerous health benefits. The Cavendish banana variety is the fifth largest agricultural commodity in the world trade after cereals, cassava, sweet potato, and yams and the fourth most important foodstuff in the world after rice, wheat, and milk [1]. Banana plays an important socioeconomic role in developing countries from tropical and subtropical zones, especially in East, Center, and West African countries, from South East of Asia, Central, and South America, and the Caribbean [2]. Cavendish banana production is currently dominated by Indonesia, with the Philippines in second place. The Philippines produced roughly 7.5 million tonnes of bananas in 2020, compared to Indonesia’s over 11 million tonnes. With 2.3, 1.5, and 1.1 million tonnes of Cavendish bananas produced in 2020, the United Nations Organization for Food and Agriculture reports that Ivory Coast, Ghana, and Cameroon are Africa’s top producers of the variety [3].
Banana is not only a rich source of vitamin A, B, and C, manganese, potassium, and fibers but also it is known that 100 g of banana includes roughly 89 kcal calories, 74 g water, 1.1 g protein, 0.3 g lipid, 21.8 g carbohydrate, 2 g fiber, 1 mg sodium, 385 mg potassium, 8 mg calcium, 30 mg magnesium, 0.4 mg iron, 22 mg phosphorous, 11.7 mg ascorbic acid, 40 µg thiamin, 70 µg riboflavin, 610 µg niacin, 80–600 µg pantothenic acid, 470 µg pyridoxine, and 23 µg folic acid [4]. Banana is a climacteric fruit that ripens quickly after harvesting. Thus, it is a perishable fruit and has a very short lifespan between harvest and the onset of deterioration [5]. It is estimated that post-harvest losses in bananas can differ based on several variables, including the variety of bananas, the storage conditions, and the handling methods. In developing countries, post-harvest loss of bananas reaches 30 % or higher because of the poor storing and handling conditions which are frequently unsatisfactory while post-harvest losses are often lower in developed countries with stronger handling and storage infrastructure, averaging around 5–10 % [3]. Many storage methods have been developed to increase the time and distance between harvest and marketing for commodities. For example, the fruit is usually harvested mature and green for commercial use, and sent chilled to the importing nations. Upon arrival at their destination, the green bananas are held in modified atmospheric rooms at the distribution centres where they are treated with chemicals like ethylene gas before selling [6]. By lowering the metabolic rate, minimizing peel greening, and preventing fruit degradation, several other preservation techniques have been investigated, such as regulated and modified atmospheres [7]. Similarly low oxygen pre-treatment for two days can prevent bananas from ripening during storage and shipping [8]. To preserve the color and texture of post-harvest bananas, chemicals are frequently used to delay ripening [9]. Edible coatings [10] and the combinations of chemical dippings with edible coatings [11] are also use. Small-scale farmers in developing countries rely on the natural ripening of bananas, which might be viewed as unsustainable and uneconomical because these processes are highly expensive for them. Because of inadequate storage and ripening conditions, many ripe bananas are lost or spoiled.
Among all the methods of fruit preservation currently in use, the use of edible coatings is a popular practice that helps preserve the nutrients of food, particularly fruits, and vegetables, and offers long shelf lives [12]. Edible coatings help preserve volatile flavour components, slow down microbial growth, delay dehydration, reduce respiration, improve textural quality, and prolong the shelf life of perishable food products [13]. The antioxidant and antifungal qualities of the coating solution give it the ability to delay ripening and senescence. According to recent studies, fruit seeds and peels may potentially have antioxidant capabilities. Examples include mango seed kernels [14] pomegranate peels [15], wampee peels [16], and grape seeds and peels [17]. In the past ten years, several researchers have suggested that citrus waste might be utilized as a natural source of antioxidants [18]. While using glycerol as a plasticizer, calcium chloride has been widely used as a preservative and firming agent for whole and fresh-cut goods. The use of calcium chloride has also been linked to fruit firmness, stress tolerance, ripening, and senescence [19]. There is little information about the combined effect of lemon peel extract, and calcium chloride on banana fruits. Therefore, the objective of this study was to find an alternative solution to extend the shelf life of bananas by using a combination of lemon peel extract, glycerol, and calcium chloride as an edible coating solution and study their effect on the physicochemical and biochemical qualities of the dessert banana fruits.
2. Materials and Methods
2.1. Chemicals and Reagents
The standards lutein, alpha-carotene, beta-carotene, sucrose, fructose, and glucose were obtained from Sigma-Aldrich; acetone, acetonitrile, n-hexane, ethanol (EtOH), methanol (MeOH), methyl tert-butyl ether (MTBE), calcium chloride (CaCl2), and glycerol were purchased from Merck (Darmstadt, Germany). H2O and acetonitrile were of liquid chromatography grade.
2.2. Plant Materials
Sample Preparation and Storage
The banana fruits were purchased fresh from a local supermarket in Germany. They were in the greenest stage conceivable, as indicated by the greenness level being 1. After being delivered to the laboratory, healthy fruits were selected, disassembled, thoroughly washed with fresh tap water, and given about two hours to dry under ambient temperature. Lemon fruits were acquired from a private plantation in Foumbot, Cameroon, which is located in the western part of the country. After removing all the fruits with physiological and physical abnormalities, such as those that were rotten and immature, the fruits were immersed for around 2 hours in water containing sodium hypochlorite (230 µl.L-1) for disinfection. After that, they were rinsed with fresh tap water and the fruit’s peels were removed using a sharp knife. They were crushed with a conventional grinder after they had completely dried in the shade, producing a lemon peel powder.
2.3. Coatings and Storage Conditions
The different coatings were made of different concentrations of lemon peel extract (0 %; 2.5 %; 5 % and 10%), calcium chloride (4 %), and glycerol (2 %). The pH of the solution was adjusted to pH 5.6 with 0.1 mol.L-1 sodium hydroxide. Bananas were dipped into the prepared coating solution for 3 min and allowed for 1 h to dry. Uncoated bananas as control samples (T0) were immersed in distilled water for the same period. The storage room was equipped with LED (light-emitting diodes, manufactured by Sylvania Luxine Plus, Erlangen, Germany) light simulating daylight light (F30W/ 865- T8). The temperature and relative humidity were controlled throughout the storage period. The temperature ranged from 19–22 °C and the relative humidity was recorded with an Efento sensor (Krakow, Poland) from 40 to 60 % and was controlled with an air humidifier (PHILIPS, Amsterdam, Netherlands). The coated and uncoated bananas were stored for a period of 13 d and another 7 d for observation, and quality parameters were recorded at 4 d intervals.
2.4. Experimental Design
Nine edible coating solutions were prepared, and the chosen concentrations were based on previous experiments; T0 = control sample with only distilled water, T1 = distilled water + glycerol (2 %), T2 = glycerol (2 %) + 2.5 % lemon peel extract, T3 = glycerol (2 %) and 5 % lemon peel extract, T4 = glycerol (2 %) + 10% lemon peel extract, T5 = 4% calcium chloride (CaCl2), T6 = glycerol (2 %) + CaCl2 (4 %), T7 = glycerol (2 %), CaCl2 (4 %) and 2.5 % lemon peel extract, T8 = glycerol (2 %), CaCl2 (4 %) and 5 % lemon peel extract, T9 = glycerol (2 %), CaCl2 (4 %), and 10% lemon peel extract. These treatments were arranged in a completely randomized design (CRD) with three replications. Thirty-six fruits were used per treatment and replicated three (3) times. Ten fruits from each replicate were used for non-destructive analyses while 26 were used for destructive analyses.
2.5. Physiological Weight Loss
The weight of each banana finger was measured using an analytical balance (Sartorius Lab Instruments, GmbH & Co. KG, Göttingen, Germany). The fruit weights were recorded at regular intervals throughout storage, and the cumulative physiological weight was determined using the formula below:
where PWL represents the physiological weight loss in percentage, IM, and FM represent the initial mass and the final mass respectively.
2.6. Firmness
The fruit ‘s texture properties were measured using the texture analyzer (Model TA- XT, Stable Micro System Ltd., Surrey GU7, UK). The data was recorded using the EXPONANT software. The firmness of the fruit´s flesh was measured by punching the sample resulting in a plot of strength versus time. Compression force measurement mode, pre-test speed of 1.5 mm.s-1, test speed of 1.0 mm.s-1, post-test speed of 10 mm.s-1, trigger-auto type 2 kg, and data acquisition speed were the working circumstances used to measure the firmness value. The accessories used were a 5 mm cylindrical probe (P/5) and a heavy platform (HDP/90). Triple readings were taken and recorded at three separate locations on the fruit while resting on the texture analyzer’s sturdy platform. The average data in Newton were used.
2.7. Color Analysis
A D90 digital Nikon camera and a 35 mm F/2 D Nikon prime lens were used for colour analysis. Digital photos were captured under controlled LED illumination using a DigiEye imaging system (VeriVide, UK) with the help of DigiEye software version 2.8.0.3. The VeriVide D65 fluorescent tubes are located in the illumination box, which is a lighting cabinet with light having similar characteristics to natural daylight. Before any measurement, the system was calibrated using a white uniform board Digitizer Calibration Pack (version number 4.0) from the Digieye service Pack. Nearly five banana fruits per replicate meaning 15 banana fruits per treatment were placed on the surface of an additional rectangular blue plate in the box. The surface of the plate containing the fruits was illuminated by the mirrors. The blue background of the collected images was removed and the fruit colors were analysed with ImageJ version 1.53K (http://imagej.nih.gov/ij) and the results were obtained in RBG (Red-Blue-Green) value and then converted into La*b* values with Python. These La*b* values determined the total colour difference using the formula below.
where: ΔL represents the change in lightness. L2* and L1* are the lightness values of the two compared samples. a2 and a1 are the red-green colour values of the two compared samples. b2 and b1 are the yellow-blue colour values of the two compared samples. The notation (+ = lighter; − = darker), (+ = redder, − = greener), (+ = yellow, − = bluer) explains the interpretation of the positive and negative changes in lightness (ΔL), red-green (Δa), and yellow-blue (Δb) colour values.
△ E = [(△L) 2 + (△ a *) 2 + (△ b *) 2] ½
ΔE = [ (L2 − L1)2 + (a2 − a1)2 + (b2 − b1)2] ½
2.8. Decay Percentage
The percentage of decay or rot was determined by visual observation. The survey excluded the rotten fruits caused by physiological and/or microbiological disorders, and the percentage was estimated.
2.9. Total Soluble sugar, Titratable Acidity, and pH
A 5 g sample of the banana pulp was prepared from 3 fruit and homogenized with ultra-turrax and 15 mL of MQ water. The plant material was centrifuged for 10 min at 3,000 rpm. Total soluble sugar was determined by a digital refractometer (Atago, Pocket Brix-Acidity Meter, Tokyo 105-0011, Japan). A volume of 45 mL of MQ water was used to dilute and homogenize 5 mL of the fruit juice. The pH was measured with a digital pH meter (EC-30-PH PHOENIX instrument from ProfiLab24 GmbH, Berlin, Germany). By titrating the diluted fruit juice using a magnetic stirrer in the presence of phenolphthalein, the titratable acidity was determined and calculated using the following formula:
Titratable Acidity (TA) = (Volume of NaOH (in mL) × 0.1 (normality of NaOH) × 0.064) / (Total juice volume in mL) × 100. Where the volume of NaOH (in mL) is the volume of sodium hydroxide solution (in milliliters) used to neutralize the acidity in the juice sample during the titration process; 0.1 is the normality of the sodium hydroxide (NaOH) solution and represents its concentration; 0.064 is the citric acid milliequivalent factor and total juice volume (in mL) is the volume of the juice sample used in the titration (before the addition of NaOH).
2.10. Sugar Analysis
Ten grams of the banana pulp of each treatment were ground, diluted, and centrifuged at 13000 rpm for 10 min. The supernatant was collected, dried by lyophilization, and kept at -4 °C for further analysis. 3The used HPLC system was equipped with an S5200 autosampler and a pump 1000 Smartline with Manager 5000 (Göbel Instrumentelle Analytik GmbH, Hallertau, Germany). The detector was an evaporative light scattering detector (ELSD; PL-ELS 2100, Polymer Laboratories GmbH, Darmstadt, Germany). The column (Grace GmbH, Germany) was Allsphere Amino 250x4.6 mm, 5 µm. MilliQ water (ultrapure water) was utilized as eluent A (20 %) and acetonitrile as eluent B (80 %). The flow rate was 1.0 mLmin-1 at 30 °C. The parameters for the ELSD were Evap:80, Neb:45, Gas:1.6, Pmt:6, Smth:1, and LED:100. The data management system was Geminix III software, version 1.10.3.9 (Göbel Instrumentelle Analytik). Sucrose, glucose, and fructose standards were used for identification and quantification.
2.11. Carotenoids Analysis
Ten grams of banana pulp were extracted using ethanol/n-hexane (4:3 v/v). After centrifugation at 13,500 rpm for 10 min., the yellow supernatant was collected and re-extracted with the same solvent until it had become colorless. The supernatants were combined and dried under reduced pressure with a rotatory evaporator. The residues were dissolved with n-hexane and redried. One hundred µL of ethyl acetate was added to the dried samples, vortexed, and centrifuged at 10,000 rpm. The analysis was carried out on the Agilent Technologies 1260 Infinity HPLC (Agilent, Waldbronn, Germany) equipped with a column size of 250x4.6 mm (RP-18; YMC CO., LTD). The eluents were methanol (eluent A: 20 %) and tert.-butyl methyl ether (eluent B: 80 %). Carotenoids were detected at 440 nm. The injection volume was10 µL and the flowrate was 1 mL.min-1. The signals were converted and recorded by OpenLab software version 2.4. Identification and quantification of individual carotenoids were carried out by comparing retention times and UV/Vis absorption with those of authentic standards.
2.12. Statistical Analysis
With treatment and storage time as sources of variation, all quantitative parameter data were subjected to a one-way ANOVA using R version 4.1.2 (2021-11-01) statistical analysis software with its cross-platform development environment called Rstudio. Data were expressed as mean plus standard deviation (SD) from the three replicates. Turkey tests, compared at p < 0.05, were used to establish the level of significance of the difference between the different treatments.
3. Results
3.1. Texture Analysis
The changes in texture or firmness of the banana fruit with and without coating during the storage period are shown in Table 1. All treatments showed comparable firmness values (p > 0.05) after one day of storage. Significant firmness changes between treatments were observed after 9 days of storage (P < 0.05). In comparison to other treatments, T2 had the highest firmness value (32.1 ± 3.2 N), indicating improved firmness preservation. The lowest value measured (2.6 ± 0.3) was observed for T9, showing a considerable loss of firmness during storage. After 13 days, there were still differences between the treatments in the firmness values, which ranged from 2.3 N to 9.6 N. However, there were no significant variations between the treatments (p > 0.05). The firmness of fruits is crucial in determining how resistant they are to mechanical injury. The different treatments of the samples affected how well the firmness was preserved during storage. The results thus demonstrate the impact of the concentration of lemon peel extract (LPE) on the firmness of bananas during storage. Bananas treated with CaCl2 alone (T5) were also firmer than untreated fruit (T0) after, 9 and 13 days of storage. This result is in line with the recent observation who reported that banana fruits treated with CaCl2 have a slower rate of deterioration and texture loss [20]. In addition, CaCl2 treatment has been proven to retain effectively the firmness of chili pepper [21]. However, others reported that CaCl2 solution accelerates the ripening process of bananas [22]. In fact, fruit softening and textural changes are brought on by the depolymerization and solubilization of cell wall constituents as well as cell structure degradation [23].
3.2. Physiological Weight Loss
Due to physiological changes and water loss, banana fruits lose weight during storage. Therefore, the influence of edible coatings on the physiological weight loss (PWL) of banana fruits during storage was studied (Figure 1). As the storage time increased, the physiological weight loss in all the treatments also increased as a result of the normal ripening process in fruits. On the first day (day 1) after coating, no (0 %) physiological weight loss was recorded in both coated and non-coated banana fruits, meaning that the application of the edible coating formulation has no immediate effect on the water content of the banana. The physiological weight loss values varied at day 5 from 2.4 % to 6.2 % where some treatments exhibited noticeable differences in PWL compared to the control T0. T1 and T9 showed higher PWL values of 6.1 % and 6.4 %, respectively, indicating that the coating might have limited effectiveness in reducing PWL at this time point, but T2 demonstrated a high reduction in PWL with a value of 4.9 %, suggesting that the coating formulation in T2 has a positive impact on preserving banana weight during this period. On day 9, all treatments displayed a noticeable reduction in PWL compared to the control T0. T2, T3, and T6 showed a high reduction in PWL with values of 5.2 %, 7.6 %, and 4.9 %, respectively. The results indicate that the effect of the edible coating becomes more evident with increasing storage time. On day 13, when all the fruits were ripe, a high value of PWL was observed in all treatments in comparison to the control T0 (2.3 %) which was already at the senescent phase. T2 continued to exhibit the highest reduction in PWL with 4.9 %. Usually, the main cause of physiological weight loss for fruit and vegetables is transpiration, which is dictated by the difference in water vapour pressure between the fruit and the atmosphere [24]. Furthermore, edible coatings act as a semipermeable barrier against oxygen, carbon dioxide, moisture, and solute movement, so lowering respiration, water loss, and oxidation reaction rate [25].
3.3. Decay Percentage
The bananas that showed decay after day 13 were counted and expressed as a percentage (Figure 2). T2 showed the lowest number of damaged bananas with a decay percentage of 2.3 % ± 0.6 %, compared to the other treatments. A low concentration of LPE (2.5 %) had a strong positive effect on the shelf life of the bananas, while a high concentration of LPE (5 and 10 %) accelerated the deterioration of the fruits e.g T3 (34.4 % ± 1.5 %) and T4 (31.3 % ± 1.5 %), respectively. Similarly, CaCl2 treatment (T5) reduced the decay of banana in comparison with the control (T0) as only 11.0 % ± 1 % of the fruits were spoiled compared to T6 (31.6 % ± 2.0 %), T7 (28 % ± 2 %), T8(19.3 % ± 2.5 %), and T9 (23.6 % ± 2.0 %). The reduction of the decay of the fruits after the application of LPE as an edible coating on the surface of fruits could be explained by the antifungal effect of lemon peel. The effect of the essential oil of lemon, mandarin, grapefruit, and orange on the growth of moulds commonly associated with food spoilage has already been shown [26].
Bananas were coated differently (see Material and Methods, experimental design) and the fruits that went bad after storage were counted and expressed as a percentage. Different letters mean that the values are significantly different (p < 0.05).
3.4. Total Soluble Sugar
The total amount of soluble sugars present in a liquid or solution is referred to as total soluble sugars, also known as the total soluble solids (TSS). The total soluble sugar (TSS, °Brix) of the coated and uncoated bananas was determined during the storage (Table 1). All treatments had TSS values between 2.1 and 2.1 % Brix after an initial storage period of 1 day. The TSS values significantly differed between the treatments after the 5-day storage period. In comparison to other treatments, T0, T1, T4, T8, and T9 exhibited higher TSS values (ranging from 3.0 to 3.4 % Brix), indicating a faster ripening of these bananas in comparison with the fruits treated differently. The TSS readings continued to deviate when the storage duration increased. TSS values for T0, T3, and T4 showed the highest values with 16.8 ± 0.2, 16.6 ± 0.1, and 16.5 ± 0.1 % Brix, respectively at day 9. Similarly, results differed significantly at day 13. TSS values for T0 were highest at 17.8 ± 0.1 % Brix, indicating that all other treatments slowed the ripening process. These results of the effect of edible coatings are similar to those obtained on strawberries [27] and bananas [28]. The treatment T2 was found to be the most effective in retaining TSS during the storage period. The effect of CaCl2 was not significant enough to maintain TSS compared to other edible coating formulations. Coating film on the surface of the banana reduces the internal respiration rate and vital processes, which reduces the ripening process and keeps the TSS value low.
3.5. Color Analysis
The La*b* colour space offers insightful information about the samples’ colour properties. In this study, the change in La*b* values over time for various treatments in comparison to the control (T0) was studied (Table 2). The value L* stands for the lightness component, whereas a* and b* stand, respectively, for the red-green and yellow-blue colour components. At day 1, all treatments showed comparable L* values in comparison to the control (T0), showing that there were no appreciable variations in the samples’ lightness. The a* and b* values were also similar to T0, indicating that there were no significant differences in the red-green and yellow-blue colour components. Day 5 analysis showed that T1 had a considerably higher a* value than T0 (p < 0.05). T1 showed a shift towards a more intense red or green hue compared to T0 with an average a* value of 91.6 ± 0.5. The L and b* values between treatments did not show any discernible variations at this time. On day 9, when compared to T0, T2 had significantly lower L levels (p < 0.05). T2 had a darker appearance than T0, with an average L value of 90.3 ± 1.3. Furthermore, b values in T2 were considerably lower than in T0 (p < 0.05) and showed a trend towards a more vivid blue or yellow colour with an average b value of 12.4 ± 0.1. The values between treatments did not show any discernible variations at this period. However, when compared to the other treatments, T6 displayed a considerably higher b value at day 9 (7.5 ± 0.1). This suggests that the yellow colour of T6 was more pronounced. However, treatment T2 was greener than the other treatments on day 13. This can be concluded by the lower a* value of T2 (6.5 ± 4.5) on day 13 in comparison to the other treatments. The CIELAB colour space’s green-red axis is represented by the* value, where negative values denote greener hues. As a result, T2 had a greener appearance on day 13 than the other treatments. The results show that T3, T4, T9, and T2 retain effectively the most chlorophyll content (green colour) of the fruit while the other coated fruits are yellow due to the carotenoid content of the fruit´s peel. The green hue of the banana peel gradually fades during ripening as a result of the thylakoid membrane breaking and the chlorophyll being degraded by chlorophyllase and oxidase enzymes, revealing yellow carotenoid pigments [29]. LPE could retain effectively the green colour of banana fruit, while the combination of LPE and CaCl2 could reduce this effect. This is well observed in T8 where we have a 5 % concentration of LPE in combination with CaCl2 (4 %), which has less effect on the retention of green colour during the storage time as compared to T3 with 5 % LPE concentration without CaCl2 which presents a stronger effect on the green colour retention. However, during storage, L values fell for both coated and uncoated samples, possibly as a result of surface moisture loss, which could have resulted in the darker hue seen [30]. Similar results were obtained in the study of an edible coating based on quince seed gum at various concentrations on banana slices at 4 °C and 40 °C [31].
Coating formulations. Coating film on the surface of the banana reduces the internal respiration rate and vital processes, which reduces the ripening process and keeps the TSS value low.
3.6. Titratable Acidity, and pH
The changes in the pH of control and treated banana fruits during the storage period were determined (Figure 3). The pH increased as the storage time increased. On day 1, there is no difference between the pH of the coated and the control fruits whose value varies between 3.7 and 3.8. By day 9 and 13, the pH values increased uniformly and T2 showed the lowest pH throughout the storage period with values of 4.3 and 4.8 on day 9 and 13 respectively, while the control sample had values of 5.2 and 6.6 on days 9 and 13 respectively. No significant differences were found in the other formulations compared to the control samples.
Titratable acidity (TA) is a measure of the acidity of a solution, expressed in terms of the amount of a strong base needed to neutralize the acid in the solution. Citric acid is one of the major organic acids in ripe banana fruits. The TA gradually increased as the storage time increased (Figure 4). After the first week of storage, the TA was not significantly different between the coated and non-coated fruits. By day 13, the values of T4, T5, T6, T7, T8, and T9 were higher than the control while T3 and T2 were lower. This result showed that formulation T2 causes the titratable acidity to remain low as in the control on day 0. The coating T2 might reduce the metabolic activity and lower the fruit respiration of banana fruit. These results are in accordance with the results obtained on bananas coated with chitosan and with chitosan in combination with gibberellic acid [25].
3.7. HPLC Sugar Analysis
Glucose, fructose, and sucrose are the main sugars in banana fruit during ripening and these sugars increase in concentration during ripening [32]. The concentration of these sugars in coated and uncoated bananas was determined during the storage period (Figure 5). In general, the sugar content increased as the storage time increased. The concentrations were not significantly different from each other up to the first 9 days of storage. By day 9, the highest sucrose concentration was in T0 (2.1 mg.g-1), T6 (2.0 mg.g-1), and T7 (1.8 mg.g-1) while the lowest was recorded in T8 (0.8) mg.g-1), and T2 (1.3 mg.g-1). The sucrose concentration of T0 dropped to 0.7 mg.g-1 at day 13 while in all the coated samples the sucrose content increased. T1, T2, and T5 showed the highest sucrose concentrations at day 13 with 2.3 mg.g-1, 2.2 mg.g-1, and 2.2 mg.g-1, respectively. The fructose concentrations were higher in most of the coated fruit as compared to the control samples. By day 9, the concentration of fructose and glucose were highest in T4 (1.2 mg.g-1; 1.2 mg.g-1), T7 (1.8 mg.g-1; 1.1 mg.g-1), T6 (1.1 mg.g-1; 1.1 mg.g-1) and T3 (1.1 mg.g-1; 1.1 mg.g-1), while the lowest content was observed in T2 (0.7 mg.g-1; 0.7 mg.g-1), and T0 had values of 0.8 mg.g-1; 0.8 mg.g-1 for fructose and glucose, respectively. T0 fructose and glucose concentrations dropped to 0.7 mg.g-1 and 0.7 mg.g-1 on day 13 while the concentration of sugars in the coated fruits continued to increase. But among the coated fruits T8 (0.9 mg.g-1; 1.1 mg.g-1), T1 (0.8 mg.g-1; 0,8 mg.g-1), and T2 (0.8 mg.g-1; 0.8 mg.g-1) contained low fructose-glucose content. A complicated regulatory process changes metabolism during banana ripening from starch synthesis to starch breakdown, resulting in the accumulation of soluble sugars, primarily sucrose, which significantly affects the taste and flavour of the fruit. This conversion of starch into sucrose appears to be responsible for sweetening the pulp and providing energy for metabolic processes that lead to the development of other quality characteristics of ripe bananas, such as colour change, synthesis of volatile compounds, and even softening of the pulp, which greatly affects the quality of the finished fruit [33]. In all the samples a high concentration of sucrose compared to the concentration of fructose and glucose was detected. In fact, during the ripening process, glucose and fructose are formed from sucrose molecules. When the climacteric peak is reached, this sucrose concentration starts decreasing under the effect of saccharolytic enzymes, while fructose and glucose concentration increase. Several authors have tracked the concentration of starch, sucrose, glucose, and fructose as well as the activities of various enzymes involved in the synthesis of sucrose [34]. In the control sample T0, a typical natural ripening is observed (Figure 5), as evidenced by degradation of sucrose at day 13, while all coated samples still show a high sucrose content at day 13. From an organoleptic point of view, the coated fruits could be sweeter, since the total content of sweet-tasting sugars is higher in the coated fruit samples, which is an important criterion for consumer acceptance.
3.8. HPLC Carotenoids Analysis
The distinctive yellow colour of ripe banana peel is solely the result of chlorophyll breakdown, which obscures the yellow hue of unripe bananas. As a result, the carotenoid content in banana changes as the fruit ripening and maturation proceeds. The concentration of carotenoids (lutein, alpha-, and beta-carotene) was analysed in the coated and non-coated bananas during the storage period (Figure 6). The concentration of carotenoids increased with increasing storage time. Lutein was the major carotenoid, followed by alpha-, and beta-carotene. A large difference in carotenoid concentration was observed particularly at day 5 of the storage time, when all the treated banana fruits had a higher lutein concentration compared to the control samples that had a concentration of 0.2 mg.g-1. The highest concentration was detected in T1 (0.3 mg.g-1), T2 (0.38 mg.g-1), T7 (0.37 mg.g-1) and T9 (0.30 mg.g-1) while the alpha- and beta- carotene content remained low. At day 9 alpha-, and beta-carotene concentration were particularly high in T2 with 0.14 mg.g-1 and 0.05 mg.g-1, respectively. In the control, beta-carotene was not detected at day 9. The highest concentration of lutein was found in the control sample T0 with 0.5 mg.g-1 at day 13. Fruit like banana produces a large amount of carotenoids, which are produced from terpenoids, as the chloroplast to chromoplast transition occurs [35]. During the ripening process, the fruit colour changes due to the production of carotenoids and the destruction of chlorophylls. As in regular ripening, the highest level of lutein content in T0 and T5 compared to the other coated samples at day 13. This could indicates that T0 and T5 are in a more advanced stage of the ripening process. Thus, coating delays the ripening of the banana.
4. Conclusions
The present results lead to the conclusion that banana fruits coated post-harvest with lemon peel extract-based formulations retain significant fruit chlorophyll content despite a reduction in fruit firmness.. The addition of CaCl2 in the formulation reduced the effect of the lemon peel extract but was not enough to be considered important. The use of a concentration of 2.5 % lemon peel extract in the formulation of an edible coating for the post-harvest preservation of bananas showed a significant retention of color, sugar, firmness and carotenoids of the fruit, which are very important parameters for quality control, as a look at the behavior of treatment T2 clearly shows throughout the storage period. On the other hand, a high percentage of lemon peel extract (here 10 % in T3) in the edible coating solution applied to the surface of bananas causes the fruit to ripen very quickly compared to the control samples. These data provide significant and helpful information for keeping the quality of bananas in the fresh produce post-harvest management. The findings of this research greatly contribute to the income improvement for farmers, processors, and distributors to market banana fruit on trade in developing and emerging countries.
Author Contributions
Eric-Ivan Ngoko Tchamba: Conceptualization, investigation, methodology, analysis, writing—original draft, writing review & editing. Wilfried Schwab: Supervision, conceptualization, investigation, methodology, analysis, resources, writing—review & editing, validation. Francois Nguetsop: Supervision, methodology, Project administration, visualization. Jean Aghofack-Nguemezi: Supervision, Project administration, conceptualization, investigation, methodology. Peter Muranuyi: conceptualization, methodology, analysis. Thorsten Tybusseck: conceptualization, methodology, Formal analysis, Software, Data curation.
Funding
This work was supported by the DAAD (Deutscher Akademischer Austauschdienst) and by the Professorship Biotechnology of Natural Products.
Acknowledgments
Thomas Hoffmann, Anja Forstner, Mechthild Mayershofer, and Sadiq Mareai are acknowledged for their technical support.
Conflicts of Interest
None.
Abbreviations
| LPE | Lemon peel extract |
References
- Picq, V.; Ramillon, J.; Balanzat, E. Swift heavy ions on polymers: Hydrocarbon gas release. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 1998, 146, 496–503. [Google Scholar] [CrossRef]
- Karamura, D.; Frison, E.; Karamura, D.A.; Sharrock, S. Banana production systems in eastern and southern Africa. Banan. Food Secur. 1998, 401–412. [Google Scholar]
- In Brief to The State of Food Security and Nutrition in the World 2023; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023. [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Dwivany, F.M.; Aprilyandi, A.N.; Suendo, V.; Sukriandi, N. Carrageenan Edible Coating Application Prolongs Cavendish Banana Shelf Life. Int. J. Food Sci. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Palta, J.P. A Postharvest Dip Treatment with of Banana Fruit. HortScience 2015, 50, 1035–1040. [Google Scholar] [CrossRef]
- Song, J.; Fan, L.; Forney, C.; Campbell-Palmer, L.; Fillmore, S. Effect of hexanal vapor to control postharvest decay and extend shelf-life of highbush blueberry fruit during controlled atmosphere storage. Can. J. Plant Sci. 2010, 90, 359–366. [Google Scholar] [CrossRef]
- Imahori, Y.; Yamamoto, K.; Tanaka, H.; Bai, J. Residual effects of low oxygen storage of mature green fruit on ripening processes and ester biosynthesis during ripening in bananas. Postharvest Biol. Technol. 2013, 77, 19–27. [Google Scholar] [CrossRef]
- Silva, V.; Arquelau, P.; Silva, M.; Augusti, R.; Melo, J.; Fante, C. Use of Paper Spray-Mass Spectrometry to Determine the Chemical Profile of Ripe Banana Peel Flour and Evaluation of Its Physicochemical and Antioxidant Properties. Quimica Nova 2020, 43, 579–585. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Fuchs, C.; Yeh, S.-Y.; Huang, F.-C.; Hoffmann, T.; Schwab, W. An oxygenase inhibitor study in Solanum lycopersicum combined with metabolite profiling analysis revealed a potent peroxygenase inactivator. J. Exp. Bot. 2010, 62, 1313–1323. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Hoffmann, T.; Schwab, W. Effects of bio-based coatings on the ripening and quality attributes of tomato (Solanum lycopersicum) fruits. J. Sci. Food Agric. 2018, 99, 1842–1849. [Google Scholar] [CrossRef]
- Ghasemzadeh, R.; Karbassi, A.; Ghoddousi, H.B. Application of Edible Coating for Improvement of Quality and Shelf-life of Raisins. World Appl. Sci. J. 2008, 3, 82–87. [Google Scholar]
- Taylor, P. Edible Films and Coatings: Tomorrow’s Packagings: A Review Edible Films and Coatings: Tomorrow ’ s Packagings: A Review. 2010, 37–41. [Google Scholar]
- Kabuki, T.; Nakajima, H.; Arai, M.; Ueda, S.; Kuwabara, Y.; Dosako, S. Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem. 2000, 71, 61–66. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Singh, R.P. Studies on Antioxidant Activity of Pomegranate (Punica granatum) Peel Extract Using in Vivo Models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Xie, H.; Hao, J.; Yang, B.; Qiu, S.; Wei, X.; Chen, F.; Jiang, Y. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2010, 118, 62–66. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2002, 87, 41–44. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N.; Grohmann, K. Biological Properties of Citrus Flavonoids Pertaining to Cancer and Inflammation. Curr. Med. Chem. 2001, 8, 135–153. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11172671. [CrossRef] [PubMed]
- Martín-Diana, A.; Rico, D.; Frías, J.; Barat, J.; Henehan, G.; Barry-Ryan, C. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 210–218. [Google Scholar] [CrossRef]
- Minh, N.P. Effectiveness of CaCl2 treatment on quality attributes of banana fruit during storage. Plant Sci. Today 2021, 9, 206–214. [Google Scholar] [CrossRef]
- Tunde-Akintunde, T. Effect of Pretreatment on Drying Time and Quality of Chilli Pepper. J. Food Process. Preserv. 2010, 34, 595–608. [Google Scholar] [CrossRef]
- Wills, R.; Tirmazi, S.; Scott, K. Effect of postharvest application of calcium on ripening rates of pears and bananas. J. Hortic. Sci. 1982, 57, 431–435. [Google Scholar] [CrossRef]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced Ripening Agents and Their Effect on Fruit Quality of Banana. Int. J. Food Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.; Jamir, A.; Ankalagi, N.; Henny, T.; Devi, O. Edible coatings in fruits and vegetables: A brief review. Pharma Innov. J. 2021, 10, 71–78. Available online: https://www.researchgate.net/profile/Manisha-Ch- Momin/publication/352994139_Edible_coatings_in_fruits_and_vegetables_A_brief_review/links/60e2f066 299bf1ea9ee13a3d/Edible-coatings-in-fruits-and-vegetables-A-brief-review.pdf.
- Gol, N.B.; Rao, T.V.R. Banana Fruit Ripening as Influenced by Edible Coatings. Int. J. Fruit Sci. 2011, 11, 119–135. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Amal, S.H.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving Strawberry Fruit Storability by Edible Coating as a Carrier of Thymol or Calcium Chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. Available online: www.idosi.org/jhsop/2(3)10/2.pdf.
- Rahman, M.; Hossain, T.; Hossain, M.; Sattar, S.; Das, P.C. Effect of banana peel extract on storage stability of banana cv. Sagar. Food Res. 2019, 4, 488–494. [Google Scholar] [CrossRef]
- Özdemir, I.S. Effect of light treatment on the ripening of banana fruit during postharvest handling. Fruits 2016, 71, 115–122. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Amraie, M.; Salehi, M. Color and weight changes of fresh-cut banana slices coated by quince seed gum: Effect of concentration, storage temperature and duration. Iran. Food Sci. Technol. Res. J. 2019, 14, 75–85. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Cordenunsi-Lysenko, B.R.; Nascimento, J.R.O.; Castro-Alves, V.C.; Purgatto, E.; Fabi, J.P.; Peroni-Okyta, F.H.G. The Starch Is (Not) Just Another Brick in the Wall: The Primary Metabolism of Sugars During Banana Ripening. Front. Plant Sci. 2019, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Wathelet, B.; Paquot, M.; Emaga, T.H. Changements texturaux et biochimiques des fruits du bananier au cours de la maturation. Leur influence sur la préservation de la qualité du fruit et la maîtrise de la maturation. Biotechnol. Agron. Soc. Environ. 2008, 12, 1335–1342. [Google Scholar]
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. Open Archive TOULOUSE Archive Ouverte (OATAO) Mechanism of Fruit Ripening. Dev. Biol. 2010, 1. [Google Scholar]
Figure 1.
Influence of different edible coating formulations on the physiological weight loss of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 1.
Influence of different edible coating formulations on the physiological weight loss of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 2.
Decay percentage of coated and uncoated bananas on day 13.

Figure 3.
Influence of different edible coating formulations on the pH of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 3.
Influence of different edible coating formulations on the pH of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 4.
Influence of different edible coating formulations on the titratable acidity of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 4.
Influence of different edible coating formulations on the titratable acidity of the banana fruits during storage at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 5.
Concentration of sugars in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 5.
Concentration of sugars in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Figure 6.
Concentration of carotenoids in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Figure 6.
Concentration of carotenoids in coated and non-coated bananas during the storage period at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).

Table 1.
Effect of edible coating formulations on the firmness, physiological weight loss (pwl), and total soluble sugar (TSS) of banana during different storage times at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
Table 1.
Effect of edible coating formulations on the firmness, physiological weight loss (pwl), and total soluble sugar (TSS) of banana during different storage times at 19–22 °C and 40–60 % relative humidity. Storage conditions T0–T9 are described in detail in the Materials and Methods section (experimental design).
| Storage period (days) | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| 1 | 38.8 ± 0.1 a | 38.2 ± 0.1 a | 38.6 ± 0.5 a | 38.5 ± 0.4 a | 38.8 ± 0.1 a | 38.5 ± 0.1 a | 38.3 ± 0.1 a | 38.5 ± 0.1 a | 38.5 ± 0.1 a | 38.3 ± 0.6 a | |
| Firmness (N) | 5 | 32.3 ± 0.8 a | 33.4 ± 1.5 a | 37.3 ± 2.4 a | 34.4 ± 3.3 a | 32.2 ± 2.2 a | 34.7 ± 2.8 a | 34.4 ± 1.7 a | 33.5 ± 0.6 a | 33.1 ± 1.3 a | 33.7 ± 3.3 a |
| 9 | 2.9 ± 0.1 a | 3.5 ± 0.1 a | 32.1 ± 3.2 b | 3.1 ± 0.4 a | 3.4 ± 0.5 a | 5.3 ± 1.4 a | 5.4 ± 2.4 a | 6.4 ± 4.9 a | 15.3 ± 19.4 ab | 2.6 ± 0.3 a | |
| 13 | 2.3 ± 0.4 a | 2.8 ± 0.3 a | 9.6 ± 1.0 a | 2.4 ± 0.4 a | 2.3 ± 0.1 a | 2.9 ± 0.3 a | 2.9 ± 0.2 a | 2.6 ± 0.5 a | 3.4 ± 0.1 a | 2.7 ± 0.4 a | |
| 1 | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | |
| TSS (%) | 5 | 3.0 ± 0.1 d | 3.0 ± 0.1 d | 2.4 ± 0.1 a | 2.7 ± 0.1 b | 3.2 ± 0.1 e | 2.7 ± 0.1 b | 2.9 ± 0.1 c | 2.7 ± 0.1 b | 3.3 ± 0.1 f | 3.4 ± 0.1 f |
| 9 | 16.8 ± 0.1 i | 13.2 ± 0.1 e | 3.3 ± 0.1 a | 16.6 ± 0.1 h | 16.5 ± 0.1 h | 13.5 ± 0.1 f | 12.6 ± 0.1 d | 11.7 ± 0.1 c | 9.6 ± 0.1 b | 15.3 ± 0.1 g | |
| 13 | 17.8 ± 0.1 f | 16.7 ± 0.2 e | 13.5 ± 0.1 a | 16.6 ± 0.1 de | 15.7 ± 0.1 b | 16.3 ± 0.1 cd | 15.9 ± 0.1 bc | 16.4 ± 0.2 de | 15.7 ± 0.2 b | 16.4 ± 0.2 de |
Note: Mean values followed by the same letter within each parameter (Firmness, PWL, and TSS) are not significantly different at p < 0.05.
Table 2.
Effect of edible coating formulations on the colour of banana storage at 19–22 °C and 40–60 % relative humidity.
Table 2.
Effect of edible coating formulations on the colour of banana storage at 19–22 °C and 40–60 % relative humidity.
| Treatments | Color (CIELab) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||
| L1* | L2* | L3* | L4* | a1* | a2* | a3* | a4* | b1* | b2* | b3* | b4* | |
| T0 | 61.8 ± 0.1 | 91.4 ± 0.9a | 91.4 ± 0.9c | 66.4a ± 0.8a | -9.8 ± 0.1 | -243.6 ± 2.9 | 14.5 ± 0.2c | 12.1 ± 0.3a | 12.0± 0.2 | 14.7 ± 0.7 a | 19.7 ± 0.3 d | 12.4±0.9a |
| T1 | 61.1 ± 0.1 | 91.5 ± 0.5a | 91.5 ± 0.5a | 68.1a ± 0.8a | -5.4 ± 0.1 | -240.27 ± 0.71 | 14.6 ± 0.8a | 13.4 ± 0.7a | 6.2 ± 0.1 | 15.2 ± 0.2 a | 18.6 ± 0.2 ad | 11.4±1.5a |
| T2 | 62.3 ± 0.1 | 90.3 ± 1.2a | 90.3 ± 1.2ab | 65.8a ± 0.4a | -8.0 ± 0.1 | -241.8 ± 1.5 | 15.1 ± 0.6ac | 11.8 ± 0.4a | 12.4 ± 0.1 | 15.4 ± 0.5 a | 17.9 ± 0.2 ab | 12.1±0.3a |
| T3 | 61.8 ± 0.1 | 92.1 ± 0.6a | 92.1 ± 0.6bc | 67.5a ± 1.4a | -7.4 ± 0.1 | -237.9 ± 0.6 | 15.3 ± 0.7ac | 12.7 ± 1.5a | 6.8 ± 0.1 | 15.1 ± 0.3 a | 19.1± 0.4 bd | 11.6±1.5a |
| T4 | 61.7 ± 0.1 | 91.4 ± 0.8a | 91.4 ± 0.8ac | 65.3a ± 0.2a | -6.8 ± 0.1 | -240.5 ± 2.6 | 15.9 ± 0.2ac | 11.8 ± 0.4a | 7.5 ± 0.1 | 15.4 ± 0.3 a | 18.2 ± 0.4 abc | 11.3±0.7a |
| T5 | 61.1 ± 0.1 | 91.8 ± 0.8a | 91.8 ± 0.8ab | 67.5a ± 1.2a | -5.4 ± 0.1 | -239.8 ± 3.5 | 15.6 ± 0.6ab | 12.0 ± 1.5a | 6.2 ± 0.1 | 14.6 ± 0.7 a | 17.7 ± 0.3 a | 12.5±1.5a |
| T6 | 61.70 ± 0.1 | 92.0 ± 0.5a | 92.0 ± 0.5ab | 68.3a ± 1.3a | -6.8 ± 0.1 | -239.5 ± 1.2 | 16.0 ± 0.4ab | 12.2 ± 1.3a | 7.5 ± 0.1 | 15.0 ± 0.2 a | 17.8 ± 0.6 a | 11.8±1.2a |
| T7 | 62.2 ± 0.1 | 92.0± 0.6a | 92.0 ± 0.6ac | 66.5a ± 2.2a | -8.0 ± 0.1 | -239.1 ± 1.9 | 16.0 ± 0.4ac | 11.0 ± 3.0a | 12.4 ± 0.1 | 15.2 ± 0.4 a | 18.8 ± 0.5ad | 10.8±0.8a |
| T8 | 61.8 ± 0.1 | 91.5 ± 0.5a | 91.5 ± 0.5ab | 69.8a ± 4.0a | -9.8 ± 0.1 | -240.4 ± 1.5 | 16.4 ± 1.0a | 13.3 ± 3.0a | 12.2 ± 0.1 | 15.3 ± 0.4 a | 18.0 ± 0.4 ab | 11.6±0.8a |
| T9 | 61.9 ± 0.1 | 91.9 ± 1.2a | 91.9 ± 1.2bc | 66.2a ± 2.4a | -5.2 ± 0.1 | -238.4 ± 2.3 | 17.1 ± 0.4bc | 12.1 ± 1.6a | 6.3 ± 0.1 | 14.6 ± 0.9 a | 19.3 ± 0.6 cd | 11.3±0.5a |
L1, a1*, and b1* represents the L, a*, and b* values on day 1; L2, a2*, and b2* on day 5; L3, a3*, b3* on day 9; L4, a4*, and b4* on day 13. Means with different superscripts are significantly different in their respective column or rows (P < 0.05). storage condition s T0–T9 are described in detail in the Materials and Methods section (experimental design).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
The Combined Effect of Lemon Peel Extract and Calcium Chloride on the Physical and Biochemical Quality Parameters of Dessert Banana (Musa acuminata var. Dwarf Cavendish) Fruit
Eric-Ivan NGOKO TCHAMBA
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









