Preprint
Review
DNA Damage Responses in Tumors Are Not Proliferative Stimuli but Rather They Are DNA Repair Actions Requiring Supportive Medical Care
Altmetrics
Downloads
124
Views
78
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Abstract
Background In tumors, somatic mutagenesis presumably drives DNA damage response (DDR) via altered regulatory pathways increasing genomic instability and proliferative activity. These considerations led to the standard therapeutic strategy against cancer: the disruption of mutation activated DNA repair pathways of tumors.
Purpose Justifying that cancer cells are not enemies to be killed, but rather they are ill human cells having the remnants of physiologic regulatory pathways.
Results 1. Genomic instability and cancer development may be originated from the flaw of estrogen signal rather than excessive estrogen signaling. 2. Healthy cells with genomic instability exhibit somatic mutations helping DNA restitution. 3. Somatic mutations in tumor cells aim the restoration of DNA damage rather than further genomic derangement. 4. In tumors, estrogen signal drives the pathways of DNA stabilization leading to apoptotic death. 5. In the peritumoral cellular infiltration, the genomic damage of tumor induces inflammatory cytokine secretion and increased estrogen synthesis. In the inflammatory cells, increased growth factor receptor (GFR) signal confers unliganded activation of estrogen receptors (ERs). 6. In tumor cells responsive to genotoxic therapy, constitutive mutations help the upregulation of estrogen signal and consequential apoptosis. In tumors non responsive to genotoxic therapy, the possibilities for ER activation via either liganded or unliganded pathway, are exhausted leading to farther genomic instability and unrestrained proliferation.
Conclusion Understanding the real character and behavior of human tumors at molecular level suggests that we should learn the genome repairing methods of tumors and follow them by supportive therapy rather than provoking additional genomic damages.
Keywords:
Subject: Medicine and Pharmacology - Oncology and Oncogenics
1. Introduction
Cancer is a complex disease presumably originating from mutations in genes promoting genomic instability and initiating cancer development [1]. In tumors, mutagenesis drives DNA damage response (DDR) via altered regulatory pathways increasing genomic instability and helping proliferative activity [2]. In tumors, altered DNA damage response serve the maintenance of survival and unrestrained proliferative activity of cells. These considerations led to the standard therapeutic strategy against cancer: the disruption of mutation activated DNA repair pathways of tumors should lead to clinical recovery of cancer patients [3]. However, the derangement of mutation driven DNA repair technique of tumors could not bridge the gap between basic research and clinical practice.
In tumors, accumulation of somatic mutations yields so called cancer driver genes and their altered regulatory protein products may manage the aggressive expansion [4]. Catalogues of genes known to be involved in cancer development were prepared by whole-exome and later whole-genome sequencing of numerous tumor samples. Analyses of thousands of cancer genomes return to a remarkably similar catalogue of around 300 genes that are mutated in at least one cancer type. Yet, many features of these mutated genes and their exact role in cancer development remain unclear. Accumulation of certain mutated genes in tumors is not enough to justify their pro-oncogenic nature.
There is a close collaboration between the activity of immune system and cancer driver mutations. The immune system has a strong impact on determining the expression of certain cancer driver genes [5]. At the same time, the appearance of certain cancer driver mutations shows correlations with the density and composition of immune competent cells in the tumor microenvironment [6]. The connection of the immune system with the appearance of cancer driver mutations is probably mediated by the fact that all somatic mutations can create neoantigens. These unknown peptides may trigger an immune response eliminating the cell that carries them; this process is known as immune-editing [5].
Cancer driver mutations influence the quantity and composition of immune cell infiltrate in the tumor microenvironment [6,7]. Somatic mutations in cancer driver genes with well known roles in immune signaling, such as CASP8 or HLA, are generally recruiting higher concentrations of immune cells into tumor microenvironment. Most likely, these pro-oncogenic mutations result in immune-evading mechanisms. By contrast, colorectal tumors with accumulated KRAS mutation, show weaker immune cell infiltrate than those without this mutation and the tumors are resistant to immune-checkpoint blockade [8].
Surprisingly, cancer driver genes are exposed even in various healthy cells exhibiting the same somatic mutations like tumors. Two studies examined somatic mutations in the entire human body [9,10]. In some individuals, cancer driver somatic mutations were found in virtually all tissues, although none of them had been diagnosed with cancer. The most interesting recent finding is the presence of somatic PTEN, KMT2D, and ARID1A mutations in healthy liver cells [11]. Hepatocytes showing these well known cancer driver mutations exhibited conspicuously increased fitness, faster expansion and regeneration under stress or other injury as compared with their counterparts without mutation.
The study on liver cells showing high fitness and regenerative capacity despite their cancer driving mutation justifies the positive impact of somatic mutations on genomic stability rather than tumor promotion. There is a plausible explanation; concentration of genome driver somatic mutations in tumors is not a pro-oncogenic effort but rather DNA stabilizer action via genomic plasticity.
Molecular cancer therapies targeting the altered DNA damage response pathways lead to continuous failures. The problem evokes that some modern cancer therapies might cause more harm than benefit as we do not exactly understand the molecular events in the background of diseases [12]. Analysis of therapeutic failures urges a complete turn in our anticancer strategy rather than farther developing and improving the families of moderately effective or even genotoxic drugs.
The aim of the present study is to justify that tumor cells are not enemies to be killed but rather ill human cells having the remnants of the same regulatory pathways like patients’ healthy cells [Compensatory]. Understanding the real character and behavior of human tumors at molecular level suggests that we should learn by watching the genome repairing methods of tumors instead of provoking additional genomic damages.
2. Use of Endocrine Disruptor Synthetic Estrogens in Human Therapy Mistakenly Strengthened the Concept of Estrogen Induced Breast Cancer
In the early 1940s, synthetic estrogens were developed for medical purposes; for the treatment of miscarriage and menopausal complaints and later for oral contraception. Diethylstilbestrol (DES) was a non steroidal hormone; ethinylestradiol (EE) was a steroidal product, while conjugated equine estrogens (CEEs) were extracted from biological samples [14].
Increased breast cancer risk in DES treated patients mistakenly suggested that synthetic estrogens activate the same subcellular pathways like high endogenous estradiol level does leading to alterations in all cellular functions, including interactions with DNA [15]. In reality, malformations and increased breast cancer risk induced by prenatal exposure to DES may be attributed to deregulation of estrogen signaling pathways. In animal experiments, DES and EE treatment provoked histone modification and further genomic damages via ER deregulation justifying their endocrine disruptor character [16].
The development of synthetic estrogens, including both DES and EE, may be regarded as pharmaceutical mistake as they are endocrine disruptors. Endocrine disruptors exhibit a special toxicological mechanism; higher doses induce more genomic damages as compared with lower doses; however, there are no safety low levels of these chemicals [17]. Low doses of synthetic estrogens exert an inhibitory effect on the ligand independent, ancient AF1 domain of ERs, while inducing compensatory estrogen-like activation on the ligand dependent AF2 domain. Conversely, high doses of synthetic estrogens provoke a serious imbalance between the liganded and unliganded activation of ERs resulting in uncompensated damages in the whole genomic machinery [18].
2.1. Synthetic Hormone Use Causes Controversial Correlations between Menopausal Hormone Therapy (MHT) and Women’s Health
For menopausal hormone therapy (MHT), both synthetic EE and CEE extracted from biological samples were prescribed [19]. From the 1940s, MHT became widely used among postmenopausal women for the treatment of menopausal symptoms and prevention of chronic illnesses, such as cardiovascular and thromboembolic complications and osteoporosis. Among postmenopausal women, the use of estrogens with different origin and even their combinations with synthetic progestins resulted in quite controversial clinical experiences concerning the risks and benefits for arterial and venous thromboembolism and female cancers, especially for the breasts. According to the guidance of Food and Drug Administration (FDA), the benefits of MHT use surpass their risks, while no comparative informations regarding the efficacy and toxicity of bioidentical versus conventional hormones could be found [19].
In the early 2000s, two great Women’s Health Initiative (WHI) studies reported quite controversial results in women underwent to MHT. In 2002, increased risks for breast cancer, thromboembolism and cardiovascular diseases were reported in menopausal women treated with conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) [20]. Conversely, in 2004, another great WHI study reported on a striking reduction of breast cancer risk in women treated with CEE (Premarin, Pfizer) alone [21]. The protective effect of Premarin with natural origin may be explained by the omission of the highly toxic progestin, MPA [22].
In 2019, a great meta-analysis study reported worldwide epidemiological evidences of the breast cancer inducing capacity of MHT independent of the used hormone formulas and timing of treatment [23]. All MHT studies reporting the breast cancer preventive effect of Premarin alone were omitted from this analysis. The concept of estrogen induced cancer was both the starting point and the goal of investigation creating a circular reasoning.
In 2020, the earlier WHI study was repeated on the survivor women eighteen years following the MHT and the results reflected long lasting breast cancer preventive effect of Premarin. Both morbidity and breast cancer associated mortality were significantly decreased among estrogen treated women [24]. These results justified long term genome stabilizer power of natural estrogen treatment without synthetic progestin use [18].
In 2021, Premarin treatment of women with ER positive, PR negative breast cancers (N=10739) resulted in significant reduction of tumors and breast cancer related deaths. Authors established, here is the time for change in their breast cancer risk reduction strategies in clinical practice [25].
Analysis of the results of MHT studies using different hormone schedules justified that horse urine derived Premarin without synthetic progestin is a highly beneficial formula against breast cancer, coronary heart disease, thromboembolism, and bone loss [22]. Although, only synthetic hormones may be blamed for breast cancer development and further complications in MHT user women, the breast cancer inducing capacity of endogenous estrogens remained evidence based fact.
2.2. Oral Contraceptives are Endocrine Disruptors Causing either Increased or Decreased Cancer Risk in Different Organs Depending on Their Regulatory Features
Oral contraceptives (OCs) comprising synthetic EE were developed in the 1960s. OCs may induce serious toxic side effects, such as venous thromboembolism, stroke and cardiovascular diseases [Lidegaard 53]. OC use induced deregulation of ER signal and led to an increased risk for insulin resistance and metabolic diseases [26].
Wide spread use of OC use among premenopausal women caused highly ambiguous correlations with cancer risk at different sites. Among OC user women, a slightly increased risk for overall breast cancer was observed [27], while strongly increased risks for ER/PR negative and triple-negative breast cancer (TNBC) were registered [28,29]. Conversely, OC use significantly reduced the risk of endometrial [30], ovarian [31] and colon cancer risk [32]. The controversial correlations between OC use and reduced or enhanced cancer risk at different sites strongly justified that ethinylestradiol is an endocrine disruptor compound rather than a bioidentical estrogen [18].
In BRCA gene mutation carriers, long term OC use significantly increases the risk for overall breast cancer as compared with non carriers [33]. Long term OC use in BRCA mutation carriers, may exert an additional inhibition on the non liganded ER activation aggravating mutation associated weakness of ERs. Conversely, in women, with BRCA1/2 gene mutations, the risk for ovarian cancer is strongly reduced by OC use [34] via exerting an advantageous estrogen-like effect by indirect activation of the AF2 domain [18].
Despite the known metabolic, thrombotic and carcinogenic complications of OCs, they are widely used in medical practice. Clinicians do not believe or do not want to believe the endocrine disruptor nature of OCs. In addition, OC use strengthened the misbelief that endogenous estrogens in higher concentrations may induce increased breast cancer risk.
3. In BRCA Gene Mutation Carrier Cells, the Defect of Liganded ER Activation is the Initiator of DNA Damage and Cancer Development
BRCA gene mutation carrier patients are pathological models for genomic instability and increased predisposition for breast and ovarian cancer development. The first breast cancer gene (BRCA1) was identified in 1994 showing close correlation with breast cancer development when becoming mutated [35], while the second breast cancer gene (BRCA2) was announced in 1995 [36]. BRCA1 and BRCA2 genes may be regarded as safeguards of the genome. Their BRCA protein products control DNA replication, transcriptional processes, DNA recombination and the repair of DNA damages [37].
Although functional BRCA proteins have crucial role in the health of all cell types in men and women, BRCA gene mutations are preferentially associated with tumor development in female breasts and ovaries [38,39].
The tissue specificity of BRCA1 mutation associated tumors suggested a potential relationship between BRCA1-loss and excessive estrogen signaling in breast cancer development. However, BRCA1 mutation linked tumors are typically ER-alpha negative, poorly differentiated and show rapid growth and poor prognosis [40]. Receptor expression profiling of BRCA1 mutant tumors showed that their vast majority proved to be ER-alpha negative and ER/PR/HER2 negative, nominated as triple negative breast cancer (TNBC) [41]. In addition, the development of ER-alpha negative breast cancer has been reported to be a predictor of BRCA1 mutation status in patients [42]. In sporadic ER-alpha negative breast cancers, reduced BRCA1 expression and decreased level of ER-alpha mRNA were parallel observed, while estrogen treatment increased BRCA1/2 mRNA levels [43]. These results suggest that BRCA gene mutation deteriorates the regulatory interplay with ERs leading to decreased ER expression and consequential decreased estrogen signal [44].
Since the regulation of female breast requires strict balance between liganded and unliganded ER activation, the weakness in ER expression and estrogen activation results in preferential susceptibility to genomic damage in the breasts of BRCA mutation carrier women [44]. In diabetes and obesity, weak estrogen signal associated defects of hormonal and metabolic equilibrium are directly associated with increased TNBC risk.
Molecular studies on interactions between BRCA1 protein and ER alpha yielded highly controversial results supporting either upregulating or downregulating effect of BRCA1 on ER alpha transactivation.
Wild type BRCA1 gene was demonstrated to inhibit ER alpha transcriptional activity under the control of its estrogen responsive elements [45]. BRCA1 could suppress the expression of near all estrogen regulated genes [46]. In addition, BRCA1 was able to inhibit p300 mediated ER acetylation, which is essential for the transactivation of ERs [47]. By contrast, it was reported that BRCA1 may induce an increased transcriptional activity of ER alpha by upregulation of p300 expression, a coactivator of ER alpha [48]. Similarly, BRCA1 ensured coactivator Cyclin D binding to ER alpha so as to facilitate the transcriptional activity [49].
The controversial findings reflect the complexity of regulatory processes including both activation and repression. In conclusion, estrogen liganded ER alpha may choose momentarily appropriate cofactors, promoter regions and transcriptional pathways in harmony with optimal BRCA1 expression and activation [50].
In genome stabilization, BRCA and ER proteins are in mutual interaction by direct binding regulating each other’s activation [51]. The amino-terminus of BRCA1 increases the activation of ER alpha, while the carboxyl-terminus of BRCA1 may function as a transcriptional repressor on ER alpha protein. ER alpha and BRCA1 are crucial components of the regulatory circuit of DNA stabilization as well [50]. Defective expression or activation of either BRCA1 or ER alpha protein disturbs their interaction, endangering both estrogen signal and genomic stability.
In women with BRCA gene mutation, anovulatory infertility frequently occurs [52] reflecting the defects of liganded estrogen signal. In addition, early menopause associated with ovarian failure is characteristic finding in BRCA mutation carriers [53]. In 85% of BRCA1 mutation carriers, loss of functional BRCA1 protein correlated with elevated aromatase levels and increased estrogen synthesis [54] suggesting compensatory actions against decreased ER expression.
In BRCA mutation carrier breast cells, decreased BRCA1 protein synthesis is associated with down-regulation of ER alpha mRNA expression and low ER alpha expression [55]. In BRCA gene mutation carrier tumor cells, a consequently decreased liganded activation of ERs was observed [45]. In BRCA gene mutation carrier breast cancer cells a decreased expression of ER alpha was experienced [56].
The defect of liganded ER activation in BRCA mutation carriers is a crucial finding as it explains the increased inclination to cancers, the ER negativity of developing tumors and the ovulatory disorders of female patients.
In BRCA Mutation Carriers, Both Healthy Cells and Tumor Cells Show Compensatory Molecular Changes Improving the Decreased Estrogen Signal
In BRCA mutation carrier women, the defect of estrogen signaling endangers the genome stability in healthy cells, and means a risk for further genomic deregulation for tumor cells. In healthy cells with BRCA mutation, a compensatory upregulation of estrogen signal may preserve genomic stability, while in BRCA mutation carrier tumor cells increased estrogen signal may protect from further genomic damage and increasing proliferative activity. Tumor cells possess the remnants of the same genome stabilizer pathways like healthy cells have. In the emergency situation of weakening estrogen signal, tumor cells may show various activating mutations increasing both liganded and unliganded ER activation [57].
Healthy cells. In mammary epithelial cells, loss of BRCA1 gene leads to increased epidermal growth factor receptor expression [58], which means an unliganded activation of ERs instead of pro-oncogenic impact. In BRCA1 mutation carrier women, BRCA1 protein activity confers the selection of appropriate CYP19 aromatase promoter region for the compensatory intensifying of estrogen synthesis [59]. In mammary fibrous adipose cells, downregulation of BRCA1 gene increased the specific activation of the PII promoter on Cyp19 aromatase gene leading to increased estrogen synthesis. Mutation of BRCA1 gene may be counteracted by unliganded activation of ERs conferred by upregulation of growth factor receptors and P13K/Akt pathways via interaction with BRCA1 protein [60].
Tumor cells. In BRCA1-deficient human ovarian cancer cells, ER alpha exhibited increased ligand independent transcriptional activity that was not observed in BRCA1 proficient cells [61]. Authors suggested that loss of BRCA1 increased unliganded ER activation; however, it was a compensatory activation attributed to the defective liganded activation
In tumor cell line with BRCA mutation, increased estrogen signal was observed via enhanced activation of p300, a transcriptional coactivator of ERs [48]. In familiar breast cancers with BRCA mutation a further transcriptional activator of ERs, Cyclin D1 was highly accumulated [62]. Nuclear factor kappaB (NF-κB), an important ER coactivator, was persistently activated in a subset of BRCA1-deficient mammary luminal progenitor cells [63].
In sporadic breast cancer cells, wild BRCA gene is capable of increasing the expression of the coding gene of ER alpha, ESR1, mediated by the activator Oct-1 [56]. Moreover, BRCA could transcriptionally increase the expression of ER alpha mRNA.
Studies on BRCA mutation carriers teach us crucial new aspects for cancer research. 1. Genome instability is linked with the weakness of liganded ER activation rather than with excessive estrogen signal. 2. BRCA gene mutation carrier healthy cells are working on the improvement of endangered DNA, via upregulation of both liganded and unliganded ER activation. 3. In BRCA mutant tumor cells, the upregulation of estrogen synthesis and unliganded ER activation are efforts protecting DNA from further damage. 4. Both healthy and tumor cells with BRCA gene mutation, exhibit gene amplification and activating gene mutations so as to increase estrogen synthesis and to improve ER activation 5. In BRCA mutation carriers, the whole body works on genome stabilization via increased ovarian and peripheral estrogen synthesis.
4. Estrogens are Principal Regulators of Genomic Machinery in Mammalian Cells
At cellular level, estrogen activated ERs (ER alpha and ER beta) are the hubs of genomic machinery orchestrating all cellular functions affecting both somatic and reproductive health [64]. Molecular players of all cellular mechanisms are recruited into regulatory circuits receiving their commands directly or indirectly from ERs and in turn, sending their signaling reports back to ERs.
DNA stabilizer circuit regulated by ER-alpha. Estrogen activated ER-alphas are the primary initiators and organizers of the regulatory circuit for DNA stabilization in a triangular partnership with genome safeguarding proteins, such as BRCA1 and aromatase enzyme (A450). The promoter regions of ESR1, BRCA1, and CYP19 aromatase genes exhibit a strong interplay for the appropriate expression of ER-alpha, BRCA1 protein and aromatase enzyme [50]. Upregulation of ER signaling is the safeguard of DNA stability in both amplifying and quenching phases of cell proliferation..
Activated ER-alpha as a transcriptional factor drives ESR1 gene expression inducing expression of protein coding ER-alpha-mRNA and ER-alpha protein. Activated ER-alphas also have the capacity to occupy BRCA1 promoter regions increasing the expression of protein-coding BRCA1 mRNA transcripts and elevated BRCA1 protein synthesis [38].
BRCA1 protein as a transcriptional factor induces the transcriptional activity of BRCA1 gene and increases BRCA1 protein expression. BRCA1 protein activates the expression of ESR1 gene and a consequential increased ER-alpha synthesis [56]. In addition, BRCA protein may occupy the CYP19A promoter region, which is BRCA1 responsive and confers an increased expression of aromatase enzyme. BRCA1 protein ensures a safety balance between the expression of ER-alpha protein and aromatase enzyme [57].
Abundant BRCA1 proteins may induce epigenetic modification and activating mutations on ESR1, BRCA1 and CYP19 aromatase genes via increasing appropriate lncRNA expression and resulting in increased production of the three regulatory proteins: ER, BRCA1 and aromatase [57]. In addition, abundant BRCA1 protein upregulates the transcriptional activity of ER-alpha conferred by either Cyclin D1 [49] or p300 coactivator protein [48]. Increased BRCA1 activity mediates a repressed unliganded activation of ERs [61], while a compensatory increase in liganded ER-activation improves DNA stability [18]. Further lncRNA transcripts of BRCA1 may stimulate amplification on CYP19 aromatase promoter gene increasing A450 aromatase enzyme expression and estrogen synthesis [59]. Increased estrogen concentrations bind and activate abundant ER-alphas, further stimulating the circuit of DNA stabilization [50].
BRCA1 and ER-alpha proteins are capable of direct binding as well; as transcriptional factors. Certain binding sites drive upregulative processes, while others may silence each other’s transcriptional activity [51]. Mutagenic alteration or low expression of ER-alpha may dangerously decrease the expression of BRCA1 mRNA transcripts and BRCA1-protein synthesis; weakening DNA-safeguarding [43]. In turn, decreased or defective synthesis of BRCA1-protein leads to downregulation of both ER-alpha mRNA expression and ER-alpha protein synthesis [55]. Loss or defect in either ER-alpha or BRCA1 protein function results in genome instability and increased cancer risk [50].
Cell proliferation circuit regulated by ER-alpha. The main regulator of cell proliferation is the estrogen activated ER-alpha in strong interplay with membrane associated tyrosine kinase growth factors receptors; epidermal growth factor receptor (EGFR), and insulin-like growth factor receptor 1 (IGF-1R) [13]. Balanced liganded and unliganded ER-alpha activation ensures a strong control over DNA replication during both increased and decreased cell proliferation. Collaboration between ER and GFR receptor families for the regulation of cell growth and proliferation may be more or less maintained even in malignant tumors [18]
IGF-1R exhibits a bidirectional signaling pathway with estrogen activated ERs [65]. IGF-I expression is regulated by insulin and growth hormone (GH), which stimulate the synthesis of IGF-I in the liver [66]. IGF-1 binding to IGF-1R activates two main signaling pathways: the phosphatidylo-inositol 3-kinase (PI3K)-AKT) and the Ras-mitogen-activated protein kinase (MAPK) pathways. These kinase cascades stimulate an unliganded transcriptional activity of ER-alpha via phosphorylation of serine residues [67].
ERs are capable of stimulating many proteins in the insulin-IGF-1 system, including IGF-1R and insulin receptor substrate 1 (IRS-1) [68,69]. ER-alpha binds and phosphorylates IGF-1R and controls its signaling pathways. In IGF-1 knock-out mice, estradiol induced uterine growth is missing [70]. In turn, in vivo IGF-1 activation of uterine cell proliferation is strongly dependent on ER-alpha activity [71].
Estrogen treatment stimulates the synthesis of EGF in uterine epithelium via ER-activation leading to proliferative effect [72]. In the absence of estrogen, EGFR signaling may be activated via unliganded ER activation [73]. Conversely, in the uterus of ER-alpha knock-out mice, EGF induced DNA synthesis and transcription was completely missing [74]. In ovariectomized mice, 17-beta-estradiol treatment caused a rapid transient upregulation of uterine EGFR mRNA and protein levels and increased the number of EGF-binding sites through ER activation [75].
In the nucleus, EGFR signal is capable of phosphorylation and activation of ER-alpha at serine 118 through the growth factor receptor activated MAPK pathway [76,77]. Phosphorylation at serine 118 increases ER-related transactivation of several genes that are upregulated by EGFR. Growth factor receptor signal may also increase the transcriptional activity of nuclear ERs via the phosphorylation of their coactivator proteins, including steroid receptor coactivator 1, p300 protein and cyclin D1 [78,79].
Cytoplasmic, estrogen-activated ERs induce an upregulation of PI3K signaling pathway via EGFR activation [80]. In endothelial cells, PI3K activation by estrogen treatment led to a rapid upregulation of 250 estrogen regulated genes within 40 minutes [81]. The ER/EGFR cross-talk at the membrane ensures the activation of multiple signaling pathways that further increases the extensive transcriptional activity of ERs [65].
In human breast cancer, the expression of ERs and EGFRs exhibit an inverse correlation [82,83]. In tamoxifen responsive breast cancer cell lines, a compensatory increased expression of ERs may be observed improving estrogen signal. In tamoxifen resistant tumors, an additional high expression of growth factor receptors may be observed [84] increasing the unliganded activation of ERs. Abundant GFRs extremely increase the unliganded activation of ERs; however, they cannot counteract the artificial blockade of AF2 domain [18].
Fuel supply circuit regulated by estrogen activated ER-alpha. Estrogen activated ER-alpha drives a regulatory circuit for the maintenance of glucose homeostasis and upregulates all steps of cellular glucose uptake providing fuel for all cellular functions [50]. Defects of eatrogen signaling lead to serious difficulties in cellular glucose uptake designated as insulin resistance and result in serious chronic diseases including cancer [85]. In conclusion, insulin resistance is the link between defective estrogen signal and increased cancer risk.
Estrogen regulated genes stimulate insulin secretion, insulin receptor expression and activation [86]. When insulin binds to insulin receptor, auto-phosphorylation of multiple tyrosines initiates the activation of insulin signal transduction [87]. Activated ERs may upregulate the expression and functional activity of intracellular glucose transporter-4 (GLUT4) facilitating insulin assisted glucose uptake [88]. ER-alpha regulates the insulin receptor substrate 1 (IRS1) mediated activation of PI3-K/mTOR signaling pathway that closes the regulatory circuit by unliganded activation of nuclear ERs [89].
Estrogen signal facilitates glucose uptake even in cancer cells providing energy for the self directed restoration of DNA stability. In MCF-7 human breast cancer cell line, estradiol increases the expression of insulin receptor substrate-1 (IRS-1) potentiating insulin signaling [90]. In ZR-75-1 breast cancer cells, estrogen/progesterone treatment upregulated glucose transporter 1 (GLUT1) expression [91]. In MCF-7 cell lines, estradiol treatment activated ERs upregulating PI3K/Akt signaling pathway parallel with a facilitated translocation of glucose transporter 4 (GLUT4) vesicles to the plasma membrane [92]. Defective or inhibited estrogen signal leads to failure of glucose uptake even in tumor cells, decreasing the possibilities for genome stabilizer processes.
5. Estrogens Are Master Regulators of Metabolism and Energy Homeostasis in the Whole Body via Orchestrating Adipose Tissue Functions
Adipose tissue deposited all over the body, provides energy and epigenetic regulatory commands for all tissues and organs via its estrogen activated ER network. In healthy adipose tissue, estrogen signal regulates the glucose homeostasis and the balance of lipolysis/lipogenesis [93,94]. In adipose tissue, damaged estrogen signal leads to defect of all regulatory functions and serious diseases may develop in the fat regulated visceral organs, cardiovascular structures and hemopoietic bone marrow [95].
The subcutaneously located adipose tissue provides energy and estrogen regulation for the skin and the skeletal muscles. Centrally positioned fatty tissue within the trunk and abdomen closely surrounds the visceral organs and cardiovascular structures, [96]. Visceral fat is largely located in the omental and mesenteric adipose tissue in the vicinity of stomach, intestines, liver and pancreas. Kidneys and the attached adrenal glands are embedded into abundant fatty tissue capsule. Adipose tissue deposition within the visceral pericardium surrounds the myocardium and coronary arteries providing estrogen signal and energy for the moving heart. Perivascular adipose tissue is nursing most blood vessels with the exception of pulmonary and cerebral arteries [97]. Further depot of adipose tissue is gonadal fat (GAT) surrounding the ovaries and testes having specific regulatory functions [98].
Female breasts enjoy exceptional nursing level as mammary lobules are intimately intermingled with the estrogen and ER rich fatty tissue pad [99]. This close connection between adipocytes and mammary cells is associated with the extreme demand of breasts for strict regulatory control and abundant energy supply. This high regulatory claim of the breasts may explain their unique vulnerability to estrogen loss or defect of ER activation.
The third largest fat depot is the bone marrow fat following subcutaneous and visceral fatty tissue. Adipocytes are active components of the bone marrow microenvironment regulating hemopoietic and immune cell proliferation and function via their estrogen signal and secretome [100].
Interestingly, the central nervous system does not enjoy the estrogen driven adipose tissue safeguard, while the brain shows extreme claim for estrogen regulation. Recently, microbial sequences were found in healthy human brain samples [101] suggesting that they may provide important support for cerebral functions. Microbiom in the gut has great role in increasing unbound, free estrogen levels via their β-glucuronidase activity [102,103]. It is a plausible possibility that gut microbiom colonized in the brain increases the level of accessible, free estrogen.
Adipose tissue is a major source of estrogen synthesis among extragonadal sites in both women and men [104]. Circulating and locally synthesized estrogen hormones regulate the functional activity of adipose tissue. Estrogens synthesized in adipose tissue are acting locally, in an autocrine manner, while increase ER activation in the adjacent organs in a paracrine manner [105]. Estrogen hormone is the main regulator of adipose tissue health via metabolic and epigenetic pathways [106]. Estrogen exerts its physiological effects on estrogen responsive adipocytes via estrogen receptors (ERα, ERβ and GPR30) [107].
In the gonads, C19 steroids are essential precursors of estrogen synthesis, while extragonadal sites are unable to synthesize estrogen directly from C19 steroids. With ageing, increased estrogen synthesis in adipose tissue requires precursor supply, such as dehydroepiandrosterone (DHEA) from external sources [108].
The noteworthy volume of ubiquitous adipose tissue and its remarkable estrogen synthesis justify that adipocytes have crucial roles in controlling and regulating the signaling network of adjacent organs and the whole body.
Secretory Functions of Visceral Adipose Tissue in Healthy Lean and Obese Patients with Defective Estrogen Signal
Abdominal adipose tissue has crucial physiological secretory functions [109]. Adipokines, cytokines and growth factors are important signaling molecules in adipose tissue and their estrogen regulated activation ensures the health of the whole body.
Sexual steroids. In adipose tissue, estrogen synthesis and appropriate estrogen signaling regulates the expression of numerous genes and the harmonized synthesis of signaling molecules [106].
Adipokines. Leptin regulates the energy balance in the hypothalamus exerting anorexinogenic and lipolytic effects. Estrogen treatment increases the expression of leptin receptors amplifying the leptin-sensitivity of various cells [110]. In estrogen deficient aromatase knock out (ARKO) mice, visceral adiposity develops and leptin levels are highly elevated [111]. Adiponectin is protective against insulin resistance mitigating various inflammatory reactions, and improving endothelial functions. Oophorectomy increases adiponectin levels in adult mice, while it may be reversed by estradiol substitution [112]. Resistin level increases parallel with obesity, which may be a compensatory reaction. In subcutaneous adipocytes, an estradiol benzoate injection decreases resistin levels [113].
Proinflammatory cytokines and low grade inflammation. Proinflammatory cytokines are regulatory proteins having great role in the maintenance of genomic and metabolic stability. In obese fatty tissue, low grade inflammatory reactions and abundantly expressed cytokines are counteractions to genomic deregulation via increasing estrogen signaling [114]. Insulin resistance of obese estrogen deficient adipose tissue leads to further regulatory disorders in the adjacent organs resulting in serious co-morbidities, such as fatty degeneration and malignancies [115,116].
In the low-grade inflammation of obese adipose tissue, increased levels of inflammatory cytokines and immune cell infiltration comprising macrophages and T cells may be found [117]. Proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) generate an increased expression and activation of aromatase enzyme resulting in increased estrogen synthesis [118]. Proinflammatory cytokines have beneficial effects against obesity and obesity related metabolic disorders via increasing aromatase activity and estrogen synthesis. Estrogen treatment of obese ovariectomized mice decreased the expression of inflammatory cytokines, included TNFα and the upregulation of estrogen signal improved the insulin sensitivity in both adipose tissue and liver [119].
Insulin-IGF system. The insulin like growth factor (IGF) system is involved in regulation and control of physiologic growth and differentiation. Insulin and insulin-like growth factor receptors act as ligand-specific modulators regulating genes on similar pathway [120]. In the early phases of insulin resistance, a compensatory increased IGF-1 level mediates increased insulin synthesis resulting in compensatory hyperinsulinemia.
Strong crosstalk and interplay between signaling pathways of ERs and growth factor receptors (IGF-1R, EGFR, VGFR) are well known in both health and disease [121,122]. Among physiological circumstances, growth factor activated ERs are capable of either stimulating or silencing cell growth and proliferation. In deregulated tumor cells, growth factor activated unliganded ERs do not turn to excessive proliferative stimulus, but rather they are initiators of DNA stabilization and apoptotic death.
Estrogens regulate both insulin like growth factor 1 (IGF-1) synthesis and the expression of its receptor (IGF-1R) in adipocytes. In turn, an increased synthesis of IGF-1 and its receptor upregulates the AKT and MAPK pathways promoting an increased unliganded activation of ERs [123]. In an estrogen deficient milieu, unliganded activation of ERs via IGF-1 receptor signaling may transiently ensure the genome wide expression of estrogen regulated genes [64]. In conclusion, in obesity and insulin resistance, increased expression and activity of IGF-1 receptors are not pro-oncogenic pathways but rather they increase unliganded ER activation.
Interaction between adipocytes and immune cells. Adipocytes are in signaling crosstalk with immune cells in both healthy and obese adipose tissue. In lean adipose tissue, IL-4 secreted by eosinophil granulocytes and regulatory T (Treg) cells activate M2 type macrophages, which express arginase and anti-inflammatory cytokines such as IL-10. In contrast, in obese adipose tissue, high number of M1 type macrophages and increased secretion of pro-inflammatory cytokines, such as TNFα and IL-6 are coupled with a decrease in anti-inflammatory immune cells [117]. In animal experiments, estrogens are capable of improving metabolic disorders and at the same time they exert anti-inflammatory effects. In female mice, estrogen protects from adipocyte hypertrophy, obesity and prevents adipose tissue oxidative stress and inflammation [124].
In obesity, upregulation of estrogen signaling restores insulin sensitivity, reduces lipid deposition, decreases pro-inflammatory cytokine synthesis and quenches inflammatory infiltration. Estrogen treatment provides quite new ways for the prevention and cure of obesity and obesity related complications.
6. Tumor Cell Itself Is the Frontline of Anticancer Combat via Increasing Estrogen Signal and Expression of Estrogen Regulated Genes
According to global medical concepts, tumor cells are enemies to be killed as they presumably fight for their survival similarly like pathogenic bacteria fight against antibiotics. Seemingly, tumor cells express cancer driver genes via somatic mutation and their altered protein products defeat both the immune defense of body and the therapeutic effect of pharmaceutical agents.
In reality, the recognition of DNA damage means an emergency state even for tumor cells. Upregulation of estrogen signal via liganded and/or unliganded pathway is the appropriate means for the restoration of DNA stability. However, in tumors, the possibility for DNA repair is questionable attributed to the genomic damage. The more differentiated a tumor, the stronger is its capacity for compensatory upregulation of estrogen signal coupled with DNA restorative efforts [125].
Spontaneous healing of early breast tumors is a well known finding justifying the capacity of initial cancers for self directed remission. A systematic review and meta-analysis study evaluated high prevalence of incidental breast cancer and precursor lesions in autopsy studies on clinically tumor-free cases. The estimated mean prevalences of incidental cancer and precursor lesion were surprisingly high: 19·5% and 0·85% [126].
Breast cancer is regarded as a multifactorial and very heterogeneous disease that refers to the abnormal proliferation of the lobular and ductal epithelium of the breast resulting in tumor formation [127].The classifications of breast cancers follow the recommendations of the World Health Organization (WHO), which are regularly revised in accordance with the scientific progress [128].
The most important parameter for the classification of breast cancers is their molecular profile, as it was described in 2000 [129]. The heterogeneity of breast cancers at molecular level was revealed through the various expression of a panel of genes. Breast cancers were divided into four main groups: 1. luminal A (60% of cases), 2. luminal B (10% of cases), 3. overexpression of human epidermal growth factor receptor 2 (HER2) (20% of cases) and 4. basal-like triple-negative breast cancers (TNBCs) (about 10% of breast cancers). Another subgroup has also been described as a normal breast-like subcategory which resembles the luminal A group but shows a worse prognosis.
In clinical practice, these tumor groups are identified by immunohistochemical markers, such as ER-alpha, progesterone (PR) and human epidermal growth factor receptor (HER2) expression [127]. In breast cancers, the overexpression of certain receptor families is mistakenly regarded as aggressive survival technique and their targeted inhibition is the principle of current therapeutic measures. In reality, missing or decreased expression of certain receptors in tumor cells highlights the points of genomic defects requiring repair. In tumors, overexpression of certain receptors and regulators, as well as activating mutation of their genes indicate the efforts for self directed genomic repair rather than developing survival techniques [13,57]. In reality, the loss of certain receptors indicates the genomic damage, while the overexpression of others represents the genome repairing effort.
Immunohistochemical markers of breast cancers show the alterations in their gene and receptor protein expression as compared with healthy breast epithelium. Molecular alterations reflecting the grade of DNA damage and the concomitant DNA repairing actions in different breast cancer types are shown by Table 1.
Luminal type A cancers are the least aggressive tumors with expression of ER alpha, and PR. Increased ER expression in breast tumors is traditionally regarded as a crucial inducer and promoter of tumor growth [127]. This concept derives from confusing the constellation with causation. Increased ER expression is not a causal factor for tumor growth but rather it is an effort for improving estrogen signal and DNA stabilization in an estrogen deficient milieu [44].
Estrogen receptor expression was shown to be parallel with DNA repair capacity in breast cancer cells [130]. This correlation justifies that high ER expression of untreated tumors is the key to self directed DNA repair, rather than a fuel for tumor growth. The strong belief in estrogen induced cancer does not allow considering opposite alternatives.
Luminal A breast cancer may exhibit transiently good response in 50% of tumors to adjuvant endocrine therapy; however, near all patients, previously showing good tumor responses later become non responders [131]. Patients with early luminal, ER-positive breast cancer are at continuous risk of relapse even after more than 10 years of tamoxifen treatment [132]. These experiences underline that endocrine disruptor therapy is not appropriate method even for early, ER positive breast cancer care.
Luminal B tumors are more aggressive than luminal A types. They express lower ER alpha and lower PR expression or may be PR negative in correlation with the weakening estrogen signal [133]. Luminal B tumors are associated with an increased rate of p53 mutations and in certain B type tumors, HER2 may also be expressed [134]. Activating p53 mutations are not oncogenic changes but rather they mean stronger DNA protection in tumors with weakening genome stability. In luminal B type tumors, appearance of HER2 expression works on the compensatory unliganded activation of ERs [18].
After tamoxifen therapy, patients with ER positive, PR negative and HER2 positive tumors exhibited higher rates of tumor recurrence and mortality as compared with those who did not receive the agent [135]. This observation suggests that in type B tumors, the wakening ER signal is further worsened by endocrine disruptor treatment. In contrast, Premarin treatment of ER positive, PR negative breast cancer cases resulted in significant reduction in tumor size and improved patients’ survival [25].
HER-2-enriched breast cancer is ER- and PR-negative and HER-2-positive. HER-2-enriched cancers tend to grow faster than luminal cancers and can have a worse prognosis. ER- and PR negativity in HER-2 enriched breast cancers reflects loss of estrogen signal and strong defects of all genomic processes. HER-2 overexpression in hormone receptor negative tumors is mistakenly regarded as a trigger for tumor proliferation similarly to all other growth factors [127]. By contrast, in the emergency situation of DNA damage, HER2 overexpression is a compensatory effort for the unliganded activation of ERs occurring scarcely in this tumor type [18]. HER-2 protein targeted therapies against HER-2-enriched tumors show similarly ambiguous results like ER inhibitor antiestrogens against ER positive tumors [13].
Triple-negative or basal-like breast cancer is ER-negative, progesterone receptor-negative, and HER-2-negative. Triple-negative breast cancer is more common in people with BRCA1 gene mutation, younger women, and black women. Triple-negative breast cancers are more aggressive than either luminal A or luminal B breast cancers and they are not responsive to endocrine therapy [127].
In triple negative breast cancers (TNBCs), the lack of ER, PR and HER-2 receptors indicate the serious deregulation of the whole genomic machinery. These tumors are poorly differentiated and clinically show rapid growth and spread. In TNBC type tumors, there is no possibility for self directed DNA repair as ERs seem to be absent or hidden and the regulatory pathways for both liganded and non liganded ER activations are unnoticeable [44]. Increased risk for TNBC type tumors in African-American women may be attributed to their excessive pigmentation in a relatively light deficient geographical region. Poor light exposure leads to metabolic and hormonal alterations conferring increased cancer risk [136].
Molecular classification of breast cancer types reflects the fact that in women, stronger estrogen signal may suppress, while a defective estrogen signal liberates breast cancer initiation and growth [44]. In tumor cells, the higher the ER expression the stronger is the apoptotic effect of therapeutic estrogen exposure. In contrast, endocrine disruptor therapies may achieve only transient tumor responses in appropriately ER positive breast cancers. Poorly differentiated ER/PR negative and TNBC type tumors are refractory to antiestrogen therapy attributed to their serious genomic deregulation.
In conclusion, breast cancers are not multifaceted tumors with quite different etiology and pathogenesis. Consequently, they do not need quite different therapies depending on their receptor status. The levels of regulatory defect create a line of variously differentiated tumors between strongly ER positive, highly differentiated and poorly differentiated TNBC type ones. In breast cancer therapy, natural estrogen is a risk free available option for ER positive tumors [25]. Against ER negative and TNBC type, poorly differentiated tumors, Maloney’s mRNA technology would be a promising therapy to be introduced in the near future [125].
7. Peritumoral Microenvironment: The Second Line of Antitumor Battle for Estrogen Regulated Genes
In the early 2000s, the role of tumor microenvironment emerged as being an important player in cancer development, tumor invasion and metastatic spread [137]. Today, cancer is regarded as a complex disease built up from the neoplastic lump and its altered cellular and stromal microenvironment [138,139]. There is a strengthening belief that tumors insidiously influence all players in their microenvironment via dynamic intercellular communication so as to help their invasive growth via escape from defensive immune reactions and anticancer treatment.
The supposed conspiration between tumors and their microenvironment is based on the belief that all signaling molecules and regulatory proteins are taken for pro-oncogenic factors when their expression is highly elevated in tumors and in the adjacent cellular infiltration [139,140,141]. In addition, when important regulatory genes, such as ESR1, are accumulated or mutated in tumors, they are regarded as pro-oncogenic alterations rather than self regulated efforts for the repair of genomic damages [142,143,144,145,146]. According to the reigning preconception, in tumor cells, the upregulation of estrogen signal and its activator pathways are regarded as keys to tumor growth.
In reality, in tumors, upregulation of certain signaling pathways and activating mutations are not pro-oncogenic factors but rather they are efforts for metabolic improvement and genomic stabilization [57]. Unfortunately, advanced tumors have weakened capacities for self directed genomic repair and they ask for help via sending messages to their microenvironment. In turn, peritumoral activated cells send signals and regulatory molecules helping the tumor to achieve DNA repair and to commit apoptosis as a kamikaze action.
Re-evaluation of studies on the biochemical and genomic communication between tumors and activated microenvironmental cells revealed that all signal messages and transported exosomes aim the upregulation of each other’s estrogen signal and improvement of all genomic functions. These activating processes serve the elimination of the tumor rather than helping its proliferation and invasion. In conclusion, the dynamic communication between the tumor and its microenvironment is a marvelous collaboration among molecular players fighting for genomic repair and apoptosis of tumor by means of their genomic plasticity.
Cancer-associated fibroblasts (CAFs) are major components emerging in the tumor microenvironment. Their assembly and activation may be attributed to signals deriving from cancer cells [138]. CAFs are in continuous signal communication with cancer cells and all other cell types in the tumor microenvironment [139]. Distant intercellular communication occurs by spherical extracellular vesicles (EVs) comprising exosomes carrying different molecules, such as proteins, DNAs, non-coding RNAs, miRNAs and mRNAs. Biochemical and genetic cross-talk between cancer cells and CAFs are important observations; however, the presumed cooperation for tumor invasion and metastatic spread is not justified, it is a biased labeling.
Activation of growth factor signaling cascades. In CAFs, the expression of growth factors, such as insulin-like growth factor (IGF-1), fibroblast growth factor FGF-7, FGF-10, HGF and TGF-beta 2 are regarded as pro-tumorigenic factors [147]. In reality, estrogen receptors and growth factor receptors are common regulators of crucial cellular functions including cell growth and apoptosis as well as metabolic processes even in tumors [65].
Transforming growth factor beta (TGF-beta) superfamily is the main inducer of CAF activation and in turn, CAFs secrete large amount of TGF-beta isoforms for improving tumor cell regulation [148]. Tumor cell-derived extracellular vesicles (EVs) may frequently contain growth factor TGF-beta, which is regarded as typical mitogen factor of tumors [149]. Considering the ER activating role of growth factors, tumors send them to CAFs for activation of their estrogen signal. Tumor derived EVs containing certain miRNAs contribute to the enhanced TGF-beta expression in CAFs through the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway [150]. PI3K and AKT/mTOR pathways upregulate ER activation and improve glucose uptake, which are not pro-tumorigenic processes, but rather increase antitumor activity. Cancer cell-derived EVs containing mRNA coding for CXCR-4 and IGF-1R provoke CAFs for growth factor secretion in acute myeloid leukemia [151].
Cytokines secreted by CAFs, macrophages and immune cells are important regulators of inflammatory processes and immune reactions in the tumor microenvironment [152]. Estrogen signal orchestrates the secretion of both pro-inflammatory and anti-inflammatory cytokines according to the momentary requirements. Pro-inflammatory cytokines stimulate aromatase activity, estrogen synthesis and ER expression in the estrogen responsive peritumoral cellular infiltration. When estrogen concentration reaches appropriately high concentration, the accumulation of anti-inflammatory cytokines will quench the inflammatory reaction parallel with the decreasing estrogen level [114].
IL-1β accumulation in hyperplastic lesions activates CAF formation from fibroblasts via NF-κB pathway [153] that is a coactivator of ERs promoting genome stabilization. Proinflammatory cytokines, IL-6 and TNF-α, are capable of aromatase activation leading to increased estrogen concentration and upregulation of estrogen signal [154]. In gastric cancer, tumor sends miRNA containing vesicles to CAFs so as to induce inflammatory cytokine/chemokine secretion through the Janus kinase (JAK)/STAT and NF-κB signaling pathways [155]. In colorectal cancers, constitutive mutation of KRAS increases the activation of EGFR kinase cascades; PI3K-Akt and RAS-RAF-MAPK, whereas increases RAS-GEF signaling pathway, which is related to abundant cytokine production [156]. In Hodgkin lymphoma, CAFs exposed to tumor cell derived EVs show increased proinflammatory cytokine secretion [157]. CAFs activated by tumor EVs, may in turn shed additional EVs that will transfer signaling and regulatory molecules to tumor cells.
Various tumors promote aromatase activity and estradiol synthesis in the peritumoral stroma via promotion of proinflammatory cytokine secretion [158]. In breast cancers, aromatase is abundantly expressed in tumor cells, intratumoral fibrous cells, and neighboring adipocytes justifying their collaboration in promotion of excessive estrogen synthesis [159]. These observations mistakenly support the role of increased estrogen concentration in tumor growth and invasion.
By contrast, a combined genetic and clinical investigation justified the anticancer capacity of increased local estrogen synthesis in tumors and their stroma. In a large prospective study, examination of the surgical breast tumor samples revealed significant correlation between low aromatase level and an increased loco-regional recurrence rate of tumors [160]. This study suggests that missing estrogen synthesis in tumors is associated with worse prognosis in breast cancer cases.
Circulating estradiol may be systemic modulator of CAF secretome as CAFs express steroid receptors [161]. Estradiol regulates the expression of several microRNAs in CAFs deriving from breast cancer [162]. In gastric cancer, estrogens stimulate IL-6 secretion of CAFs promoting signal transducer and activator of transcription (STAT-3) expression [163]. Increased expression of STAT3 in CAFs secretome confers an effort for genome stabilization as STAT3 is a transcription factor having important role in DNA replication.
Few studies evaluated growth factors and cytokines as positive regulators of the genome rather than pro-tumorigenic factors. TGF-beta was considered as a tumor suppressor factor due to its cytostatic effect on cancer cells [164]. IL-11 was known for its capacity to stimulate platelet production in cancer patients with thrombocytopenia [165].
Immune cells in the tumor microenvironment show intense interaction with tumor cells. The interaction between immune cells and other cell types are regulated by cell surface immune checkpoints [138]. Mast cells are recruited near tumors during tumorgenesis and release a variety of cytokines and chemokines [166]. Cytokines and chemokines are crucial regulators of both genomic and immunologic processes and their accumulation is an anticancer effort. Natural killer cells (NK) are cytotoxic and secrete tumor necrosis factor so as to kill tumor cells [167].
Tumor-associated macrophages (TAMs) infiltrate the microenvironment of tumors and are mainly divided into two categories: classically activated macrophages (M1 type) and alternatively activated macrophages (M2 type). Activated M2 type macrophages are blamed for managing immune escape of tumors. The abundance of TAM infiltration in tumors is mechanically linked with poor disease prognosis [168]. TAM activation and accumulation in tumors is not a pro-oncogenic feature, but rather their intense cytokine secretion is helping aromatase activity and increasing estrogen concentration.
Myeloid-derived suppressor cells (MDSC) have apparently immunosuppressive effects; they may block immunotherapy and may play a role in tumor maintenance and progression [169]. MDSCs also accumulate in response to the chronic inflammation and lipid deposition in obesity and contribute to the more rapid progression of cancers in obese individuals. In reality, accumulation of MDSCs is not a causal factor of rapid tumor progression and obesity associated inflammation but rather it seems to be an intense immune defense against metabolic disorder associated tumors.
Tumor infiltrating lymphocytes (TILs) are important participants of the tumor microenvironment [152]. Immune cell infiltrates may exhibit ambiguous properties either promoting or inhibiting tumor progression depending on the features of primary tumor [170]. CD4+ T cell polarization has been identified as a mediator of tumor immune surveillance. T helper 1 (Th1) cell functions are associated with tumor suppression and upregulation of IFNγ and IL-12. T helper 2 (Th2) responses are reliant of IL-4 production and presumably exhibit tumor promoting activity [171,172]. Murine and human studies reported that increased E2 concentration induces increased Th2 responses and upregulate IL-4 secretion [173,174].
A remarkable fact that constellation of strong estrogen signal and increasing tumor growth does not justify causal correlation. A recent study reported increased immune cell infiltrate comprising Th1 T cells, B cells, and cytotoxic T lymphocytes (CTLs) in ER-negative breast tumors as compared with ER-positive cancers [175]. Correlation between ER-negative breast tumors and more intensive immune cell infiltration strongly suggest that poorly differentiated tumors with loss of estrogen signal need stronger immune support for their DNA repair than highly differentiated ER-positive ones.
Gene expression analysis in ER-positive breast cancer patients showed that blocking the liganded ER activation with aromatase inhibitor (letrozole) continuously increased the tumor infiltration with B cell and T helper lymphocyte subsets following treatment initiation [158]. This result justified that letrozole inhibition of estrogen signal in ER-positive tumors induced an emergency state promptly recruiting strong immune cell infiltration.
In conclusion, tumors and their microenvironment are allies in the fight against worsening genomic defects and consequential tumor invasion. The more serious the genomic damage of a tumor, the denser is the peritumoral immune cell infiltration attributed to the emergency state. Invasive tumor spread coupled with intensive peritumoral cellular infiltration may be regarded as a common failure of tumor and peritumoral cells rather than the victory of presumably conspirator partners.
8. Molecular Changes in Tumors Responsive and Non Responsive to Endocrine Therapy Reflect the Successful or Unsuccessful Counteraction to ER Blockade
The traditional belief of estrogen induced breast cancer required the introduction of inhibitors of estrogen signal for breast cancer care. The pharmaceutical industry developed two kinds of antiestrogens for therapeutic purpose: a selective estrogen receptor modulator, tamoxifen and an aromatase inhibitor (AI), letrozole [176]. Since the early 1970s, antiestrogens are commonly used compounds for breast cancer care as adjuvant therapy.
Antiestrogen therapy of patients with breast cancer yielded many difficulties from the onset attributed to the development of so called endocrine resistance of tumors. Antiestrogen treatment could not surpass the “magic” 30% of tumor response rate, similarly to the weaknesses of other endocrine therapies; such as oophorectomy or high dose synthetic estrogen treatment [177]. The majority of overall breast cancers (≥70%) were not responsive to antiestrogen therapy exhibiting stagnation or even a rapid growth. In addition, about a half of the targeted ER-positive tumors showed primary resistance to antiestrogen therapy [131]. Moreover, near all patients showing earlier good tumor responses to endocrine treatment later experienced secondary resistance leading to metastatic disease and fatal outcome [178].
In the past decades, great efforts were exerted for revealing the mechanism of presumed endocrine resistance of ER-positive breast cancers so as to predict responses to adjuvant endocrine therapy in patients. Researchers mistakenly supposed that both responsive and non responsive tumor cells are aggressive enemies developing various techniques fighting for their survival [13].
8.1. Successful Fight of Antiestrogen Responsive Tumors against the Endocrine Disruptor Treatment
In antiestrogen responsive tumors, the principal action against the blockade of AF2 domain is a compensatory restoration and upregulation of liganded ER activation [57].
1.Tamoxifen treatment facilitates the prompt compensatory unliganded activation of ERs via translocation of ER-alphas out of the nucleus to membrane associated EGFRs [179] (Figure 1.). 2. Long term endocrine therapy upregulates the expression of the most studied coactivator of ER-alpha; AIB1 (amplified in breast cancer 1) [180]. Under tamoxifen treatment, another ER coactivator, cyclin D1 increases the activation of both STAT3 and ERs [181]. 3. Tamoxifen extremely activates the transcription factor NFκB and its upregulative crosstalk with ER-alpha [182,183]. 4. Tamoxifen provokes increasing expression of certain microRNAs that bind to mRNAs of ERs and activate the translational processes [184]. 5. Tamoxifen increases the copy number of ESR1 gene coupled with an increased expression and activation of ERs [185,186] (Figure 2). 6. AI treatment induces an acquired amplification of aromatase encoding CYP19A1 gene enhancing both enzyme expression and estrogen synthesis [187]. 7. In antiestrogen treated tumor cells, copious lncRNA transcripts of ERs confer activating mutations for crucial genes participating in the genome stabilizer circuit; such as ESR1, BRCA1 and CYP19A [57].
In breast cancers responsive to antiestrogen treatment, the facilitated regulatory processes promote the compensatory restoration of liganded ER activation and achieve a successful tumor response [188].
8.2. Unsuccessful Fight of Tumors Becoming Non Responsive to Endocrine Disruptor Treatment
When an earlier antiestrogen responsive breast cancer exhausted the possibilities for liganded ER-activation, the upregulation of unliganded ER-activation through growth factor receptor signal remains as an ultimate refuge for DNA stabilization [18]. However, even an extreme increase in unliganded ER activation is incapable of restoring ER signaling when the liganded pathway is completely blocked (Figure 3.).
In tumors non responsive to antiestrogens, there are physiological pathways for increasing unliganded ER-activation. In tamoxifen resistant tumors, an increased expression of ER coactivator HOXB7, enhances kinase domain phosphorylation of both EGFR [189] and HER2 [190] promoting the unliganded activation of ERs. Estrogen receptor coactivator AIB1 and HER2/neu signaling stimulates hormone independent ER activation [191]. In tumor xenografts, both ER and HER2 activations were associated with the compensatory upregulation of MUCIN4 [192]. In endocrine resistant tumors, an increased expression of either EGFRs [193] or IGF-1Rs [194,195] at the plasma membrane, amplifies the unliganded activation of ERs.
In endocrine resistant tumors, acquired mutations may highly increase the compensatory unliganded activation of ERs.
1.ER mediated mutation of ERBB2 gene of growth factor receptor tyrosine kinases increases the expression and activity of growth factor receptors conferring unliganded activation for ERs [191]. 2. In endocrine refractory ER-positive breast cancer, PIK3CA gene is frequently mutated upregulating the components of the PI3K-AKT-mTOR pathway and increasing unliganded ER activation [196]. 3. AI resistant breast cancers frequently exhibit acquired point mutations in the ligand binding domain (LBD) of ESR1 gene conferring a constitutive hormone independent activation of ERs [197]. 4. In antiestrogen resistant tumors, chromosomal rearrangement affecting ESR1 gene is a further mutational mechanism driving an increased unliganded activation of ERs [198]. 5. In tamoxifen-resistant breast cancer cells, activation of PI3K/AKT pathway is associated with the significant upregulation of BARD1 and BRCA1 protein expressions through an increased unliganded activation of ERs [199].
9. Estrogen Treatment Restores Genomic Regulation and Induces Apoptotic Death in Antiestrogen Resistant Tumors
Estrogen treatment of breast cancers resistant to either long term estrogen deprivation (LTED-R) or tamoxifen (TAM-R) triggers an apoptotic death in tumors [200,201]. Considering the strong upregulation of both ER and GFR expressions in breast cancers unresponsive to antiestrogen treatment, estrogen is capable of exerting its physiological anticancer capacity via a balanced liganded and unliganded activation of abundant ERs. In reality, estrogen does not restore the “antiestrogen sensitivity” of unresponsive breast cancer, but rather helps tumor cells to overcome the poisonous medicament.
Important lessons may be drawn from the 50 years of breast cancer therapy with antiestrogens. 1. In tumors, there is no endocrine therapy resistance but rather the possibilities for compensatory ER activation are exhausted. 2. In tumors responsive to antiestrogen therapy, increased ER expression and activation is not a survival technique but rather it is an effort for increasing estrogen signaling. 3. In tumors non responsive to antiestrogen therapy, increased growth factor receptor expression and activation is not a survival technique but rather it is an effort for compensatory unliganded ER activation. 4. Tumors exhaustively treated by aromatase inhibitor, show genomic plasticity exhibiting acquired mutations on the ligand binding domain of ESR1 gene conferring new, hormone-independent activation of modified ERs in the absence of estrogen.
10. Conclusions
From various organs, female breasts exhibit unique sensitivity to genomic instability caused by either germline or acquired gene mutations. This fact may partially explain that breast cancer has become the flagship of cancer research. Although, the old preconception of estrogen induced breast cancer led breast cancer care to a quite erroneous pathway, thorough examination of the controversies between estrogen signal and cancer development yielded valuable progress in overall cancer research.
Correlation between genomic instability and conspicuously increased breast cancer risk in germline BRCA gene mutation carriers revealed that the defect of genome stabilizer circuit is the origin of cancer initiation rather than excessive estrogen signals. Defect of ER, BRCA or aromatase enzyme upsets the triangular partnership of these regulatory proteins leading to weakness of estrogen signaling and genomic instability. BRCA mutation carrier healthy and tumor cells similarly show efforts for increasing the liganded and unliganded ER activation and for compensatory upregulation of another genome safeguarding protein, p53.
Understanding the fight of cancer cells for activation of estrogen signaling together with genome stabilization reveals the secret of various receptor landscapes of breast cancer subtypes. In tumors, the increased expression of hormone receptors reflects efforts for increasing liganded ER activation, while the overexpression of HER2 means trying to increase unliganded ER activation. The blockade of either ERs or HER2s seems to be an erroneous therapeutic concept. Breast cancers are not resistant to genotoxic therapies but rather they exhausted all possibilities for defending the remnants of genomic stability. Progressive genomic instability leads to unrestrained proliferative activity.
Cellular infiltration of the tumor microenvironment is not organic part of tumors. Inflammatory cells are recruited by the tumor itself and the intercellular communication by messages and extracellular vesicles confer asking help. The stronger the genomic deregulation in the tumor, the denser is the adjacent infiltration of activated mesenchymal and immune competent cells. Immune competent cells do not need therapeutic genomic machination as they exactly know their task in the anticancer fight. When tumor invasion is coupled with dense peritumoral infiltration, supportive genome repairing therapy is necessary rather than the disruption of mutation activated DNA repair pathways of tumor.
In conclusion, the improvement of genomic stability may be the new strategy in cancer therapy. Upregulation of estrogen signal leads to strengthened immune response, whilst induces apoptotic death of tumors in a Janus faced manner.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Klapp, V.; Álvarez-Abril, B.; Leuzzi, G.; Kroemer, G.; Ciccia, A.; Galluzzi, L. The DNA Damage Response and Inflammation in Cancer. Cancer Discov 2023, 13, 1521–1545. [Google Scholar] [CrossRef]
- Sinkala, M. Mutational landscape of cancer-driver genes across human cancers. Sci Rep 2023, 13, 12742. [Google Scholar] [CrossRef] [PubMed]
- Porta-Pardo, E.; Valencia, A.; Godzik, A. Understanding oncogenicity of cancer driver genes and mutations in the cancer genomics era. FEBS Lett. 2020, 594, 4233–4246. [Google Scholar] [CrossRef] [PubMed]
- Marty, R.; Kaabinejadian, S.; Rossell, D.; Slifker, M.J.; van de Haar, J.; Engin, H.B.; de Prisco, N.; Ideker, T.; Hildebrand, W.H.; Font-Burgada, J.; et al. MHC-I genotype restricts the oncogenic mutational landscape. Cell 2017, 171, 1272–1283.e15. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Overman, M.J.; Boutin, A.T.; Shang, X.; Zhao, D.; Dey, P.; Li, J.; Wang, G.; Lan, Z.; Li, J.; et al. KRAS IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019, 35, 559–572.e7. [Google Scholar] [CrossRef]
- García-Nieto, P.E.; Morrison, A.J.; Fraser, H.B. The somatic mutation landscape of the human body. Genome Biol 2019, 20, 298. [Google Scholar] [CrossRef]
- Yizhak, K.; Aguet, F.; Kim, J.; Hess, J.M.; Kübler, K.; Grimsby, J.; Frazer, R.; Zhang, H.; Haradhvala, N.J.; Rosebrock, D.; et al. (2019) RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 2019, 364, eaaw0726. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, T.; Jia, Y.; Luo, X.; Gopal, P.; Li, L.; Odewole, M.; Renteria, V.; Singal, A.G.; Jang, Y.; et al. Somatic mutations increase hepatic clonal fitness and regeneration in chronic liver disease. Cell 2019, 177, 608–621.e12. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? Int. J. Mol. Sci. 2022, 23, 13217. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Compensatory estrogen signal is capable of DNA repair in antiestrogen-responsive cancer cells via activating mutations. J of Oncol 2020; Article ID 5418365.
- Coelingh-Bennink, H.J.; Verhoeven, C.; Dutman, A.E.; Thijssen, J. The use of high-dose estrogens for the treatment of breast cancer. Maturitas 2017, 95, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Rogan, E.; Cavalieri, E. Mechanism of metabolic activation and DNA adduct formation by the human carcinogen diethylstilbestrol: The defining link to natural estrogens. Int J Cancer 2009, 124, 1276–84. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi-Clarke, L.; de Assis, S.; Warri, A. Exposures to Synthetic Estrogens at Different Times During the Life, and Their Effect on Breast Cancer Risk. J Mammary Gland Biol Neoplasia. 2013, 18, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Rasanayagam, S.; Engel, C.; Rizzo, J. State of the evidence 2017: an update on the connection between breast cancer and the environment. Environ Health 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Amplified crosstalk between estrogen binding and GFR signaling mediated pathways of ER activation drives responses in tumors treated with endocrine disruptors. Recent Pat Anticancer Drug Discov 2018, 13, 428–44. [Google Scholar] [CrossRef]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med 2005; 118(Suppl. 12B): 64-73.
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L. , et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–33. [Google Scholar]
- Anderson, G.L.; Limacher, M.; Assaf, A.R. , et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–12. [Google Scholar]
- Suba, Z. Synthetic Estrogens Deregulate Estrogen Receptors Inducing Thromboembolic Complications and Cancer. In: Topics in Anti-Cancer Research. Vol. 8. Eds: Atta-ur-Rahman and Khurshid Zaman. Bentham Science Publishers 2019, Chapter 2, pp. 44-73. [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Aragaki, A.K.; Manson, J.E.; Stefanick, M.L.; et al. Association of Menopausal Hormone Therapy with Breast Cancer Incidence and Mortality during Long Term Follow-up of Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Pan, K. Breast Cancer Prevention: Time for Change. JCO Oncol Pract 2021, 17, 709–716. [Google Scholar] [CrossRef]
- Cortés, M.E.; Alfaro, A.A. The effects of hormonal contraceptives on glycemic regulation. Linacre Q 2014, 81, 209–18. [Google Scholar] [CrossRef]
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 2017, 377, 2228–39. [Google Scholar] [CrossRef]
- Rosenberg, L.; Boggs, D.A.; Wise, L.A.; Adams-Campbell, L.L.; Palmer, J.R. Oral contraceptive use and estrogen/progesterone receptor-negative breast cancer among African American women. Cancer Epidemiol Biomarkers Prev 2010, 19, 2073–9. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Sullivan-Halley, J. , et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res 2010, 70, 575–87. [Google Scholar] [CrossRef]
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. The Lancet Oncology 2015; 16( 9): 1061–70.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008, 371, 303–14. [Google Scholar] [CrossRef]
- Bosetti, C.; Bravi, F.; Negri, E.; La Vecchia, C. Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis. Hum Reprod Update 2009, 15, 489–98. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Qi, X.; Wang, Q. Long term use of oral contraceptives comprising synthetic estrogens induces an excessive breast cancer risk in BRCA mutation carrier women: a meta-analysis. Clinical and Experimental Obstetrics & Gynecology 2022, 49, 1. [Google Scholar]
- Huber, D.; Seitz, S.; Kast, K.; Emons, G.; Ortmann, O. Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review. Arch Gyn Obst 2020, 301, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef]
- Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–92. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef]
- Gorski, J.J.; Kennedy, R.D.; Hosey, A.M.; Harkin, D.P. The complex relationship between BRCA1 and ERalpha in hereditary breast cancer. Clin Cancer Res 2009, 15, 1514–8. [Google Scholar] [CrossRef]
- Wang, L.; Di, L.J. BRCA1 and estrogen/estrogen receptor in breast cancer: where they interact? Int J Biol Sci 2014, 10, 566–75. [Google Scholar]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J. , et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002, 20, 2310–8. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R. , et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001, 98, 10869–74. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Stefansson, I.M.; Chappuis, P.O. , et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003, 95, 1482–5. [Google Scholar] [CrossRef]
- Spillman, M.A.; Bowcock, A.M. BRCA1 and BRCA2 mRNA levels are coordinately elevated in human breast cancer cells in response to estrogen. Oncogene. 1996, 13, 1639–45. [Google Scholar]
- Suba, Z. Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: preventive and therapeutic implications. OncoTargets Ther 2014, 7, 147–64. [Google Scholar] [CrossRef]
- Fan, S.; Wang, J.; Yuan, R. , et al. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 1999, 284, 1354–6. [Google Scholar]
- Xu, J.; Fan, S.; Rosen, E.M. Regulation of the estrogen-inducible gene expression profile by the breast cancer susceptibility gene BRCA1. Endocrinology 2005, 146, 2031–47. [Google Scholar]
- Ma, Y.; Fan, S.; Hu, C.; Meng, Q.; Fuqua, S.A.; Pestell, R.G.; et al. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Molecular Endocrinology 2010, 24, 76–90. [Google Scholar] [CrossRef]
- Fan, S.; Ma, Y.X.; Wang, C. , et al. p300 Modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res 2002, 62, 141–51. [Google Scholar]
- Wang, C.; Fan, S.; Li, Z.; Fu, M.; Rao, M.; Ma, Y.; et al. Cyclin D1 antagonizes BRCA1 repression of estrogen receptor alpha activity. Cancer research 2005, 65, 6557–67. [Google Scholar] [CrossRef]
- Suba, Z. DNA stabilization by the upregulation of estrogen signaling in BRCA gene mutation carriers. Drug Des Devel Ther 2015, 9, 2663–2675. [Google Scholar]
- Fan, S.; Ma, Y.X.; Wang, C.; Yuan, R.Q.; Meng, Q.; Wang, J.A.; Erdos, M.; Goldberg, I.D.; Webb, P.; Kushner, P.J.; Pestell, R.G.; Rosen, E.M. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 2001, 20, 77–87. [Google Scholar] [CrossRef]
- Oktay, K.; Kim, J.Y.; Barad, D.; Babayev, S.N. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol 2010, 28, 240–4. [Google Scholar] [CrossRef]
- Lin, W.T.; Beattie, M.; Chen, L.M.; Oktay, K.; Crawford, S.L.; Gold, E.B.; Cedars, M.; Rosen, M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer 2013, 119, 1652–9. [Google Scholar]
- Chand, A.L.; Simpson, E.R.; Clyne, C.D. Aromatase expression is increased in BRCA1 mutation carriers. BMC Cancer. 2009, 9, 148. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. Toward a unified concept of mammary carcinogenesis Aldaz M. C. Gould M. N. McLachlan J. Slaga T. J. eds. Progress in Clinical and Biological Research: 1-16, Wiley-Liss New York 1997.
- Hosey, A.M.; Gorski, J.J.; Murray, M.M.; Quinn, J.E.; Chung, W.Y.; Stewart, G.E.; et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst 2007, 99, 1683–94. [Google Scholar] [CrossRef]
- Suba, Z. Activating mutations of ESR1, BRCA1 and CYP19 aromatase genes confer tumor response in breast cancers treated with antiestrogens. Recent Pat Anticancer Drug Discov 2017, 12, 136–147. [Google Scholar] [CrossRef]
- Burga, L.N.; Hu, H.; Juvekar, A.; et al. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res 2011, 13, R30. [Google Scholar] [CrossRef]
- Ghosh, S.; Lu, Y.; Katz, A.; Hu, Y.; Li, R. Tumor suppressor BRCA1 inhibits a breast cancer-associated promoter of the aromatase gene (CYP19) in human adipose stromal cells. Am J Physiol Endocrinol Metab 2007, 292, 246–252. [Google Scholar] [CrossRef]
- Ma, Y.; Hu Ch Riegel, A.T.; Fan, S.; Rosen, E.M. Growth factor signaling pathways modulate BRCA1 repression of estrogen receptor-alpha activity. Mol Endocrinol 2007, 21, 1905–23. [Google Scholar] [CrossRef]
- Zheng, L.; Annab, L.A.; Afshari, C.A.; Lee, W.H.; Boyer, T.G. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci USA. 2001, 98, 9587–92. [Google Scholar] [CrossRef]
- Arnold, A.; Papanikolaou, A. Cyclin D1 in Breast Cancer Pathogenesis. J Clin Oncol 2005, 23, 4215–24. [Google Scholar] [CrossRef]
- Sau, A.; Lau, R.; Cabrita, M.A.; Nolan, E.; Crooks, P.A.; et al. Persistent Activation of NF-κB in BRCA1-Deficient Mammary Progenitors Drives Aberrant Proliferation and Accumulation of DNA Damage Cell Stem Cell 2016, 19, 52-65.
- Maggi, A. Liganded and unliganded activation of estrogen receptor and hormone replacement therapies. Biochim Biophys Acta 2011, 1812, 1054–1060. [Google Scholar] [CrossRef]
- Levin, E.R. Bidirectional Signaling between the Estrogen Receptor and the Epidermal Growth Factor Receptor. Mol Endocrinol 2003, 17, 309–317. [Google Scholar] [CrossRef]
- Ohlsson, C.; Mohan, S.; Sjögren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef]
- Martin, M.B.; Franke, T.F.; Stoica, G.E.; Chambon, F.; Katzenellenbogen, B.S.; Stoica, B.A.; et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 2000, 141, 4503–11. [Google Scholar] [CrossRef]
- Chan, T.W.; Pollak, M.; Huynh, H. Inhibition of insulin-like growth factor signaling pathways in mammary gland by pure antiestrogen ICI 182,780. Clin Cancer Res 2001, 7, 2545–54. [Google Scholar]
- Lee, A.V.; Jackson, J.G.; Gooch, J.L.; Hilsenbeck, S.G.; Coronado-Heinsohn, E.; Osborne, C.K.; et al. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol 1999, 13, 787–96. [Google Scholar] [CrossRef]
- Sato, T.; Wang, G.; Hardy, M.P.; Kurita, T.; Cuncha, G.R.; Cooke, P.S. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology 2002, 143, 2673–9. [Google Scholar] [CrossRef]
- Klotz, D.M.; Hewitt, S.C.; Ciana, P.; Raviscioni, M.; Lindzey, J.K.; Foley, J.; et al. s. Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross talk. J Biol Chem 2002, 277, 8531–7. [Google Scholar] [CrossRef]
- DiAugustine, R.P.; Petrusz, P.; Bell, G.I.; Brown, C.F.; Korach, K.S.; McLachlan, J.A.; et al. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 1988, 122, 2355–63. [Google Scholar] [CrossRef]
- Vignon, F.; Bouton, M.M.; Rochefort, H. Antiestrogens inhibit the mitogenic effect of growth factors on breast cancer cells in the total absence of estrogens. Biochem Biophys Res Commun 1987, 146, 1502–8. [Google Scholar] [CrossRef]
- Curtis, S.W.; Washburn, T.; Sewall, C.; DiAugustine, R.; Lindzey, J.; Couse, J.F.; et al. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 1996, 93, 12626–30. [Google Scholar] [CrossRef]
- Das, S.K.; Tsukamura, H.; Paria, B.C.; Andrews, G.K.; Dey, S.K. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology 1994, 134, 971–81. [Google Scholar] [CrossRef]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–4. [Google Scholar] [CrossRef]
- Bunone, G.; Briand, P.A.; Miksicek, R.J.; Picard, D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J 1996, 15, 2174–83. [Google Scholar] [CrossRef]
- Zwijsen, R.M.; Buckle, R.S.; Hijmans, E.M.; Loomans, C.J.; Bernards, R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev 1998, 12, 3488–98. [Google Scholar] [CrossRef]
- McMahon C, Suthiphongchai T, DiRenzo J, Ewen ME. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA 1999, 96, 5382-7.
- Razandi, M.; Pedram, A.; Parks, S.; Levin, E.R. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 2003, 278, 2701–12. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Hughes, C.C.W.; Levin, E.R. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology J Biol Chem 2002, 277, 50768–75. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Chrysogelos, S.A. Identification and characterization of a negative regulatory element within the epidermal growth factor receptor gene first intron in hormone-dependent breast cancer cells. J Cell Biochem 2002, 85, 601–14. [Google Scholar] [CrossRef] [PubMed]
- deFazio, A.; Chiew, Y.E.; Sini, R.L.; Janes, P.W.; Sutherland, R.L. Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer 2000, 87, 487–98. [Google Scholar] [CrossRef]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–33. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Interplay between insulin resistance and estrogen deficiency as co-activators in carcinogenesis. Pathol Oncol Res 2012, 18, 123–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate-2 induction in ovariectomized diabetic rats. Endocrinology 2005, 146, 4786–94. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, M.; Hedo, J.A.; Yamada, K.M.; Kahn, C.R. The structure of insulin receptor and its subunits. Evidence for multiple non reduced forms and a 210,000 possible proreceptor. J Biol Chem. 1982, 257, 10392–9. [Google Scholar]
- Campello, R.S.; Fátima, L.A.; Barreto-Andrade, J.N.; Lucas, T.F.; Mori, R.C.; Porto, C.S.; Machado, U.F. Estradiol-induced regulation of GLUT4 in 3T3-L1 cells: involvement of ESR1 and AKT activation. J Mol Endocrinol 2017, 59, 257–268. [Google Scholar] [CrossRef]
- Richards, R.G.; DiAugustine, R.P.; Petrusz, P.; Clark, G.C.; Sebastian, J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci USA. 1996, 93, 12002–7. [Google Scholar] [CrossRef]
- Mauro, L.; Salerno, M.; Panno, M.L.; Bellizzi, D.; Sisci, D.; Miglietta, A. , et al. Estradiol increases IRS-1 gene expression and insulin signaling in breast cancer cells. Biochem Biophys Res Commun. 2001, 288, 685–9. [Google Scholar] [CrossRef]
- Medina, R.A.; Meneses, A.M.; Vera, J.C.; Guzman, C.; Nualart, F.; Astuya, A. , et al. Estrogen and progesterone up-regulate glucose transporter expression in ZR-75-1 human breast cancer cells. Endocrinology. 2003, 144, 4527–35. [Google Scholar] [CrossRef]
- Garrido, P.; Morán, J.; Alonso, A.; González, S.; González, C. 17β-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology 2013, 154, 1979–89. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Mahboobifard, F.; Pourgholami, M.H.; Jorjani, M.; Dargahi, L.; et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother 2022, 156, 113808. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Low estrogen exposure and/or defective estrogen signaling induces disturbances in glucose uptake and energy expenditure. J Diabet Metab 2013, 4, 272–81. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Rajsheker, S.; Manka, D.; Blomkalns, A.L.; Chatterjee, T.K.; Stoll, L.L.; Weintraub, N. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 2010, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Alonso, J.; Al-Massadi, O.; Castelao, C.; Peinado, J.R.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. J. Proteom. 2011, 74, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Leclercq, G. Recent insights into the effect of natural and environmental estrogens on mammary development and carcinogenesis. The Int J of Develop Biol 2011, 55, 869–78. [Google Scholar] [CrossRef]
- Wang, H.; Leng, Y.; Gong, Y. Bone Marrow Fat and Hematopoiesis. Front. Endocrinol 2018, 9, 694. [Google Scholar] [CrossRef]
- Link, C.D. Is There a Brain Microbiome? Neuroscience Insights. 2021; 16. [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S. , et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem 2019, 294, 18586–99. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–96. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Luu-The, V. , et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998, 63, 322–328. [Google Scholar] [CrossRef]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.Å.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front Endocrinol (Lausanne) 2022, 13, 828780. [Google Scholar] [CrossRef]
- Dieudonné, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol-Cell Physiol 2004, 286, C655–C661. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J Am Acad Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Wang, P.; Mariman, E.; Renes, J.; Keijer, J. The Secretory Function of Adipocytes in the Physiology of White Adipose Tissue. J Cell Physiol 2008, 216, 3–13. [Google Scholar] [CrossRef]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006, 55, 978–987. [Google Scholar] [CrossRef]
- Simpson, E.R.; Misso, M.; Hewitt, K.N. , et al. Estrogen—the good, the bad, and the unexpected. Endocrine Reviews 2005, 26, 322–330. [Google Scholar]
- Combs, T.P.; Pajvani, U.B.; Berg, A.H. , et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004, 145, 367–383. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S. , et al. The hormone resistin links obesity to diabetes. Nature. 2001, 409, 307–12. [Google Scholar] [CrossRef]
- Suba, Z. Crossroad between obesity and cancer: a defective signaling function of heavily lipid laden adipocytes (Online First). In: Crosstalk in Biological Processes. Ed: El-Esawi MA. InTechOpen, London, May 3rd 2019. 3 May. [CrossRef]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular mechanisms underlying metabolic syndrome: the expanding role of the adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol Cells 2014, 37, 365–371. [Google Scholar] [CrossRef]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Research 2002, 4, 65. [Google Scholar] [CrossRef]
- Ye, J.; McGuinness, O.P. 2013. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab 2013, 304, E466–E477. [Google Scholar] [CrossRef]
- Boucher, J.; Tseng, J.-H.; Kahn, C.R. Insulin and insulin-like growth factor receptors act as ligand-specific amplitude modulators of a common pathway regulating gene. J Biol Chem 2010, 285, 17235–17245. [Google Scholar] [CrossRef]
- Smith, C.L. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 1998, 58, 627–32. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, Q.; Lin, G.; Fu, X.; Zhou, K.; Jiang, P. , et al. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1α to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep 2012, 39, 8177–85. [Google Scholar] [CrossRef] [PubMed]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V. , et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc Natl Acad Sci USA 2014, 111, 4892–7. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes, Obesity and Metabolism. 2012, 14, 58–66. [Google Scholar] [CrossRef]
- Suba, Z. Rosetta Stone for Cancer Cure: Comparison of the Anticancer Capacity of Endogenous Estrogens, Synthetic Estrogens and Antiestrogens. Oncol Rev 2023, 17, 10708. [Google Scholar] [CrossRef]
- Thomas, E.T.; Mar, C.D.; Glasziou, P.; Wright, G.; et al. Prevalence of incidental breast cancer and precursor lesions in autopsy studies: a systematic review and meta-analysis. BMC Cancer. 2017, 17, 808. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int J Mol Sci 2023, 24, 6834. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R. , et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology. 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. , et al. Molecular Portraits of Human Breast Tumours. Nature. 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Matta, J.; Morales, L.; Ortiz, C.; Adams, D.; Vargas, W.; Casbas, P. , et al. Estrogen Receptor Expression Is Associated with DNA Repair Capacity in Breast Cancer. PLoS One 2016, 11, e0152422. [Google Scholar] [CrossRef]
- Hayes DF: Tamoxifen:, Dr. Jekyll and Mr. Hyde? J Natl Cancer Inst 2004, 96, 895–897. [Google Scholar]
- Munzone, E.; Colleoni, M. Optimal management of luminal breast cancer: how much endocrine therapy is long enough? Ther Adv Med Oncol. 2018, 10, 1758835918777437. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Weiss, H.; Lee, A.V.; Schiff, R.; De Placido, S.; Osborne, C.K.; et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 2005, 97, 1254–61. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z. , et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, P.; Roger, P.; Rousseau, E.; Thibault, S.; Romieu, G. , et al. HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: results from a French regional cohort. Cancer Med. 2014, 3, 134–42. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Light deficiency confers breast cancer risk by endocrine disorders. Recent Pat Anticancer Discov 2012, 7, 337–44. [Google Scholar] [CrossRef]
- Chew, V.; Toh, H.; Abastado, J. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. Journal of Oncology 2012, 2312956. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Linares, J.; Marín-Jiménez, J.A.; Badia-Ramentol, J.; Calon, A. Determinants and Functions of CAFs Secretome during Cancer progression and Therapy. Front Cell Dev Biol 2021, 8, 621070. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 2022 Nov;21(11):799-820.
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Pejerrey, S.M.; Dustin, D.; Kim, J.A.; Gu, G.; Rechoum, Y. , et al. The impact of ESR1 mutations on the treatment of metastatic breast cancer. Horm Cancer 2018, 9, 215–28. [Google Scholar] [CrossRef]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V. , et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc Natl Acad Sci USA 2014, 111, 4892–7. [Google Scholar] [CrossRef]
- Lei, J.T.; Gou, X.; Seker, S.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat. 2019, 5, 38. [Google Scholar] [CrossRef]
- Stellato, C.; Porreca, I.; Cuomo, D.; Tarallo, R.; Nassa, G.; Ambrosino, C. The "busy life" of unliganded estrogen receptors. Proteomics. 2016, 16, 288–300. [Google Scholar] [CrossRef]
- Fan, P.; Jordan, V.C. New insights into acquired endocrine resistance of breast cancer. Cancer Drug Resist 2019, 2, 198–209. [Google Scholar] [CrossRef]
- Yu, B.; Wu, K.; Wang, X.; Zhang, J.; Wang, L.; Jiang, Y. , et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis 2018, 9, 1082. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–98. [Google Scholar] [CrossRef]
- Giusti, I.; Francesco, M.; Di D'Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G. , et al. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. Ther. 2018, 19, 722. [Google Scholar]
- Dai, G.; Yao, X.; Zhang, Y.; Gu, J.; Geng, Y.; Xue, F. , et al. Colorectal cancer cell–derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bull. Cancer 2018, 105, 336–49. [Google Scholar] [CrossRef]
- Huan, J.; Hornick, N.I.; Shurtleff, M.J.; Skinner, A.M.; Goloviznina, N.A.; Roberts, C.T. , et al. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res. 2013, 73, 918–929. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int J Mol Sci 2018, 19, 611. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 2010, 17, 135–47. [Google Scholar] [CrossRef]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Research 2002, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yamamoto, Y.; Sakamoto, N.; Shimomura, I.; Kogure, A.; Kumazaki, M. , et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene 2019, 38, 5566–5579. [Google Scholar] [CrossRef]
- Meng, M.; Zhong, K.; Jiang, T.; Liu, Z.; Kwan, H.Y.; Su, T. The current understanding on the impact of KRAS on colorectal cancer. Biomedicine & Pharmacotherapy 2021, 140, 111717. [Google Scholar]
- Dörsam, B.; Bösl, T.; Reiners, K.S.; Barnert, S.; Schubert, R.; Shatnyeva, O. , et al. Hodgkin lymphoma-derived extracellular vesicles change the secretome of fibroblasts toward a CAF phenotype. Front. Immunol. 2018, 9, 1358. [Google Scholar] [CrossRef]
- Dannenfelser, R.; Nome, M.; Tahiri, A.; Ursini-Siegel, J.; Vollan, H.K.M.; Haakensen, V.D.; Helland, A.; Naume, B.; Caldas, C.; Borresen-Dale, A.L. , et al. Data-driven analysis of immune infiltrate in a large cohort of breast cancer and its association with disease progression, er activity, and genomic complexity. Oncotarget. 2017, 8, 57121–33. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miki, Y.; Akahira, J.I.; Moriya, T.; Ohuchi, N.; Sasano, H. Review: Aromatase in human breast carcinoma as a key regulator of intratumoral sex steroid concentrations. Endocrine J 2008, 55, 455–63. [Google Scholar] [CrossRef] [PubMed]
- Bollet, M.A.; Savignoni, A.; De Koning, L.; Tran-Perennou, C.; Barbaroux, C.; Degeorges, A. , et al. Tumor aromatase expres sion as a prognostic factor for local control in young breast cancer patients after breast-conserving treatment. Breast Cancer Res 2009, 11, R54. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Ghosh, S.; Procopio, M.G.; Mazzeo, L.; Bordignon, P.; Ostano, P.; et al. (2018). Androgen receptor functions as transcriptional repressor of cancer-associated fibroblast activation. J. Clin. Invest. 128, 5465–5478.
- Vivacqua, A.; Muoio, M.G.; Miglietta, A.M.; Maggiolini, M. (2019). Differential microRNA landscape triggered by estrogens in cancer associated fibroblasts (CAFs) of primary and metastatic breast tumors. Cancers 2019, 11, 412. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, X.; Li, Z.; Xue, Y. Estrogen facilitates gastric cancer cell proliferation and invasion through promoting the secretion of interleukin-6 by cancer-associated fibroblasts. Int. Immunopharmacol. 2020, 78, 105937. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev Drug Discov 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Xu, L.; Dai, Y.; Teng, Y.; Ma, S.; et al. Multicenter, randomized study of genetically modified recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in cancer patients receiving chemotherapy. Support. Care Cancer 2012; 20, 1875–1884.
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: facilitators of cancer and obesity-induced cancer. Annu Rev Cancer Biol. 2021, 5, 17–38. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Haabeth, O.A.; Lorvik, K.B.; Hammarstrom, C.; Donaldson, I.M.; Haraldsen, G.; Bogen, B.; Corthay, A. Inflammation driven by tumour-specific th1 cells protects against b-cell cancer. Nat. Commun. 2011, 2, 240. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. Cd4(+) t cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Fish, E.N. The x-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2015, 6, 635. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M. , et als. Association between cd8+ t-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–43. [Google Scholar] [CrossRef]
- Bentrem, D.J.; Jordan, V.C. Role of antiestrogens and aromatase inhibitors in breast cancer treatment. Curr Opin Obstet Gynecol. 2002, 14, 5–12. [Google Scholar] [CrossRef]
- Jordan, V.C.; Lewis-Wambi, J.S.; Patel, R.R.; Kim, H.; Ariazi, E.A. New hypotheses and opportunities in endocrine therapy: amplification of oestrogen-induced apoptosis. Breast. 2009; 18 Suppl 3: S10-7.
- Osborne, C.K. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998, 339, 1609–18. [Google Scholar] [CrossRef]
- Fan, P.; Agboke, F.A.; Cunliffe, H.E.; Ramos, P.; Jordan, V.C. A molecular model for the mechanism of acquired tamoxifen resistance in breast cancer. Eur J Cancer. 2014, 50, 2866–76. [Google Scholar] [CrossRef]
- Tilghman, S.L.; Sabnis, G.; Brodie, A.M.H. Upregulation of AIB1, aromatase and ERα provides long-term estrogen-deprived human breast cancer cells with a mechanistic growth advantage for survival. Horm Mol Biol Clin Investig. 2011, 3, 357–366. [Google Scholar] [CrossRef]
- Ishii, Y.; Waxman, S.; Germain, D. Tamoxifen Stimulates the Growth of Cyclin D1–Overexpressing Breast Cancer Cells by Promoting the Activation of Signal Transducer and Activator of Transcription 3. Cancer Res 2008, 68, 852–60. [Google Scholar] [CrossRef]
- Zhou, Y.; Yau, C.; Joe WGray, J.W.; Chew, K.; Dairkee, S.H.; Moore, D.H. , et al. Enhanced NFκB and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007, 7, 59. [Google Scholar] [CrossRef]
- Frasor, J.; El-Shennawy, L.; Stender, J.D.; Kastrati, I. NFκB Affects Estrogen Receptor Expression and Activity in Breast Cancer through Multiple Mechanisms. Mol Cell Endocrinol. 2015; 418(0 3): 235–239.
- Hayes, E.L.; Lewis-Wambi, J.S. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015, 17, 40. [Google Scholar] [CrossRef]
- Holst, F.; Stahl, P.R.; Ruiz, C.; Hellwinkel, O.; Jehan, Z.; Wendland, M. , et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet 2007, 39, 655–60. [Google Scholar] [CrossRef]
- Tomita, S.; Zhang, Z.; Nakano, M.; Ibusuki, M.; Kawazoe, T.; Yamamoto, Y.; Iwase, H. Estrogen receptor alpha gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci. 2009, 100, 1012–17. [Google Scholar] [CrossRef]
- Magnani, L.; Frige, G.; Gadaleta, R.M.; Corleone, G.; Fabris, S. , et al. Acquired CYP19A1 amplification is an early specific mechanism of aromatase inhibitor resistance in ER alpha metastatic breast cancer. Nat Genet 2017, 49, 444–50. [Google Scholar] [CrossRef]
- Suba, Z. The pitfall of the transient, inconsistent anticancer capacity of antiestrogens and the mechanism of apparent antiestrogen resistance. Drug Des Devel Ther. 2015, 9, 4341–53. [Google Scholar] [CrossRef]
- Jin, K.; Kong, X.; Shah, T.; Penet, M.-F.; Wildes, F.; Sgroi, D.C. , et al. The HOXB7 protein renders breast cancer cells resistant to tamoxifen through activation of the egfr pathway, Proc. Natl. Acad. Sci. 2012, 109, 2736–41. [Google Scholar] [CrossRef]
- Jin, K.; Park, S.; Teo, W.W.; Korangath, P.; Cho, S.S.; Yoshida, T. , et al. HOXB7 is an ERA cofactor in the activation of HER2 and multiple er target genes leading to endocrine resistance. Cancer Discov 2015, 5, 944–59. [Google Scholar] [CrossRef]
- Osborne, C.K.; Bardou, V.; Hopp, T.A.; Chamness, G.C.; Hilsenbeck, S.G. , et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 2003, 95, 353–61. [Google Scholar] [CrossRef]
- Chen, A.C.; Migliaccio, I.; Rimawi, M.; Lopez-Tarruella, S.; Creighton, C.J.; Massarweh, S.; et al. Upregulation of MUCIN4 in ER-positive/HER2-overexpressing breast cancer xenografts with acquired resistance to endocrine and HER2-targeted therapies. Breast Cancer Res. Treat. 2012; 134 (2) 583–593.
- Fan, P.; Wang, J.; Santen, R.J.; Yue, W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 2007, 67, 1352–60. [Google Scholar] [CrossRef]
- Song, R.X.; Barnes, C.J.; Zhang, Z.; Bao, Y.; Kumar, R. , et al. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA 2004, 101, 2076–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Moerkens, M.; Ramaiahgari, S.; de Bont, H.; Price, L.; Meerman, J.; et al. Elevated insulin-like growth factor-1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res 2011, 13, R52. [Google Scholar] [CrossRef]
- Ellis, M.J.; Perou, C.M. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov 2013, 3, 27–34. [Google Scholar] [CrossRef]
- Pejerrey, S.M.; Dustin, D.; Kim, J.A.; Gu, G.; Rechoum, Y.; et al. The impact of ESR1 mutations on the treatment of metastatic breast cancer. Horm Cancer 2018, 9, 215–28. [Google Scholar] [CrossRef]
- Lei, J.T.; Gou, X.; Seker, S.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat. 2019, 5, 38. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Zhang, C.; Chu, J.; Wu, Y.; Yudong Li, Y.; et al. Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat Commun. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C.; Fan, P.; Abderrahman, B.; Maximov, P.Y.; Hawsawi, Y.M.; Bhattacharya, P.; et al. Sex steroid induced apoptosis as a rational strategy to treat anti-hormone resistant breast and prostate cancer. Discov Med. 2016, 21, 411–27. [Google Scholar] [PubMed]
- Mansouri, S.; Farahmand, L.; Hosseinzade, A. ; Eslami-SZ; Majidzadeh-AK Estrogen can restore Tamoxifen sensitivity in breast cancer cells amidst the complex network of resistance. Biomedicine & Pharmacotherapy 2017, 93, 1320–1325. [Google Scholar]
Figure 1.
Rapid response to Tamoxifen (T) induced ER blockade in cancer cells. Rapid translocation of unbound estrogen receptors (ERs) out of the nucleus helps their interactions with membrane associated growth factor receptors; GFRs (IGF1-R, EGFR). Cytoplasmic ERs activated by growth factor receptors initiate rapid transcriptional processes in the nucleus via transcriptional factors (TFs). Growth factor (GF) activated GFRs may also induce unliganded activation on nuclear unbound ERs driving their transcriptional activity. E: estrogen, P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.
Figure 1.
Rapid response to Tamoxifen (T) induced ER blockade in cancer cells. Rapid translocation of unbound estrogen receptors (ERs) out of the nucleus helps their interactions with membrane associated growth factor receptors; GFRs (IGF1-R, EGFR). Cytoplasmic ERs activated by growth factor receptors initiate rapid transcriptional processes in the nucleus via transcriptional factors (TFs). Growth factor (GF) activated GFRs may also induce unliganded activation on nuclear unbound ERs driving their transcriptional activity. E: estrogen, P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.
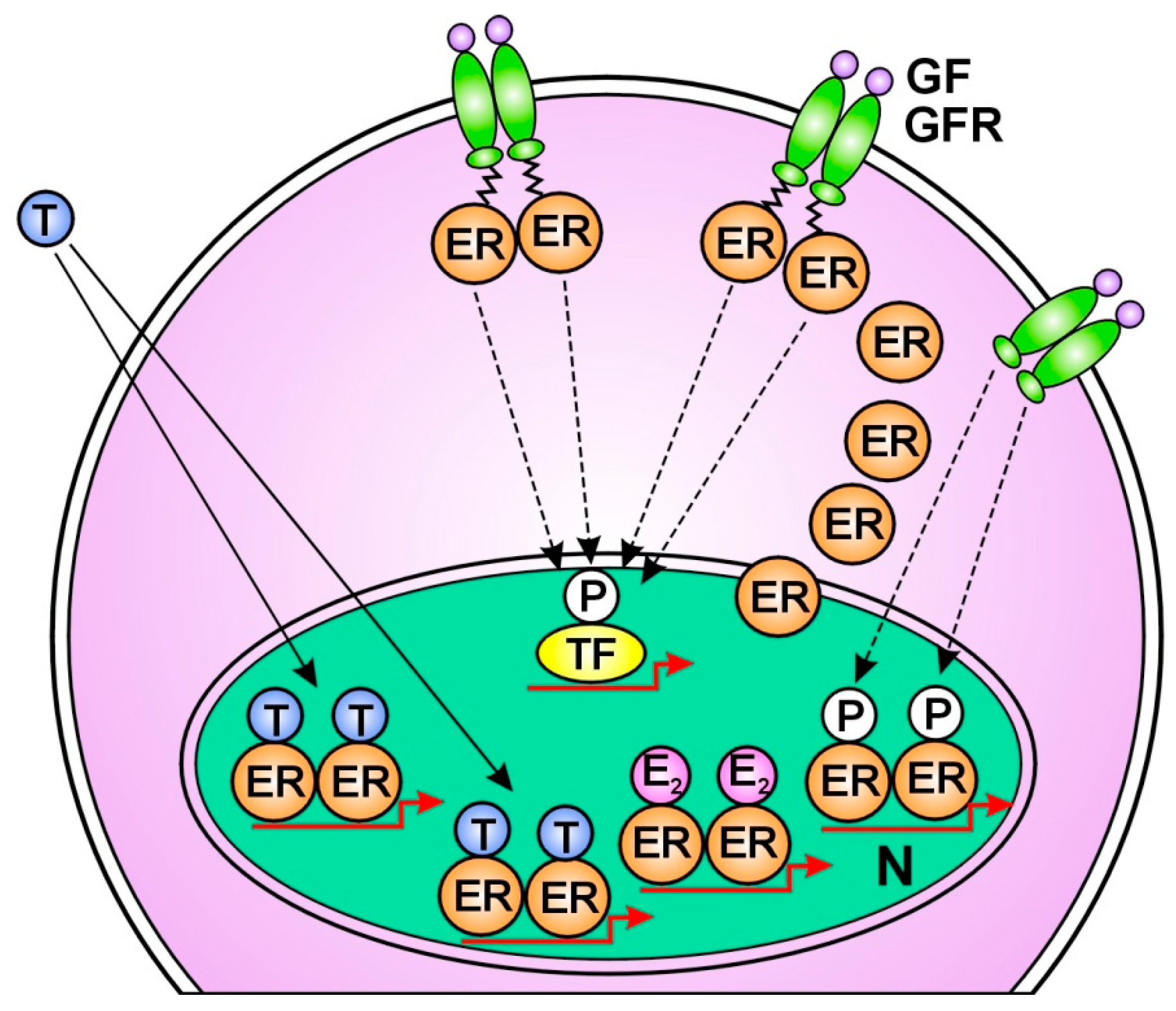
Figure 2.
Molecular mechanism of tumor response in Tamoxifen (T) treated cancer cells. Increased estradiol (E2) concentration activates newly expressed abundant estrogen receptors (ERs) increasing the expression of estrogen regulated genes. In the meantime, growth factors (GFs) activate growth factor receptors (GFRs) conferring unliganded activation for free nuclear ERs. The predominance of estradiol (E2) bound ERs over T bound ones leads to DNA repair, apoptotic death and clinical tumor response. P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.
Figure 2.
Molecular mechanism of tumor response in Tamoxifen (T) treated cancer cells. Increased estradiol (E2) concentration activates newly expressed abundant estrogen receptors (ERs) increasing the expression of estrogen regulated genes. In the meantime, growth factors (GFs) activate growth factor receptors (GFRs) conferring unliganded activation for free nuclear ERs. The predominance of estradiol (E2) bound ERs over T bound ones leads to DNA repair, apoptotic death and clinical tumor response. P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.
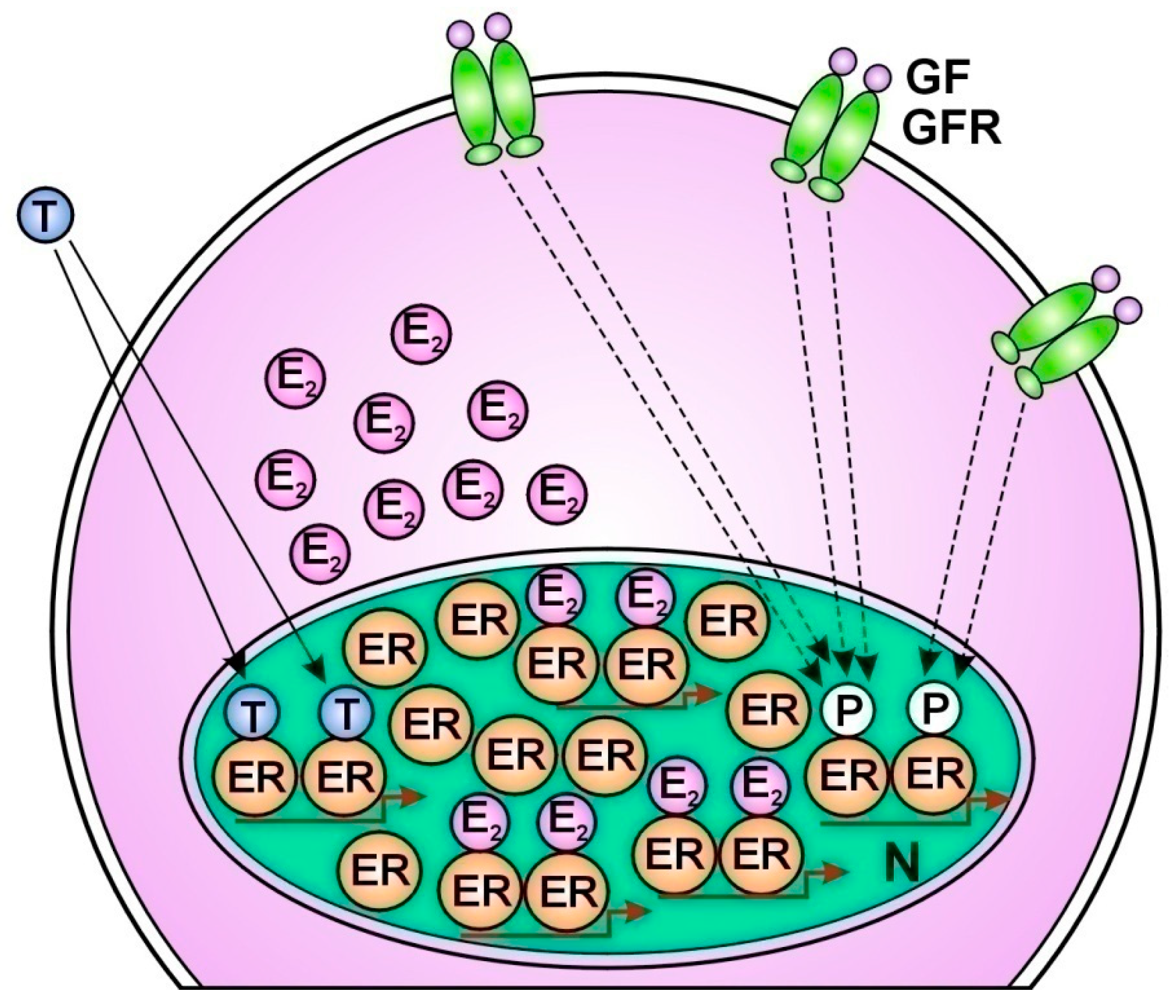
Figure 3.
Molecular mechanism of tumor resistance in Tamoxifen (T) treated cancer cells. The liganded activation of abundant nuclear estrogen receptors (ERs) is completely blocked by T binding. Compensatory abundant expression of membrane associated growth factor receptors (GFRs) struggles for the unliganded activation of T bound ERs. However, the T blockade inhibits the restoration of ER signaling resulting in unrestrained proliferation. GF: growth factor, N: nucleus, Running arrow: inhibition.
Figure 3.
Molecular mechanism of tumor resistance in Tamoxifen (T) treated cancer cells. The liganded activation of abundant nuclear estrogen receptors (ERs) is completely blocked by T binding. Compensatory abundant expression of membrane associated growth factor receptors (GFRs) struggles for the unliganded activation of T bound ERs. However, the T blockade inhibits the restoration of ER signaling resulting in unrestrained proliferation. GF: growth factor, N: nucleus, Running arrow: inhibition.

Table 1.
Receptor pattern in breast cancer subtypes reflecting the grade of DNA damage and the concomitant actions for DNA repair.
Table 1.
Receptor pattern in breast cancer subtypes reflecting the grade of DNA damage and the concomitant actions for DNA repair.
| Subtype of breast cancer | Receptor status | Signs of DNA damage | Signs of DNA repair | Proliferative activity | Response to endocrine therapy |
|---|---|---|---|---|---|
| Luminal A type (50-60%) |
ER overexpression PR positive |
no | ER overexpression | low | good in 50% |
| Luminal B type (10%) |
ER positive PR pos/neg HER2 pos/neg |
PR negative | PR negative PR positive HER2 positive |
increased | moderate/inverse |
| HER2 Enriched (20%) |
ER negative PR negative HER2 rich |
ER negative PR negative |
HER2 rich | high | no |
| Triple negative (10%) |
ER negative PR negative HER2 negative |
ER negative PR negative HER2 negative |
no | high | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
07 March 2024
Posted:
07 March 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
07 March 2024
Posted:
07 March 2024
You are already at the latest version
Alerts
Abstract
Abstract
Background In tumors, somatic mutagenesis presumably drives DNA damage response (DDR) via altered regulatory pathways increasing genomic instability and proliferative activity. These considerations led to the standard therapeutic strategy against cancer: the disruption of mutation activated DNA repair pathways of tumors.
Purpose Justifying that cancer cells are not enemies to be killed, but rather they are ill human cells having the remnants of physiologic regulatory pathways.
Results 1. Genomic instability and cancer development may be originated from the flaw of estrogen signal rather than excessive estrogen signaling. 2. Healthy cells with genomic instability exhibit somatic mutations helping DNA restitution. 3. Somatic mutations in tumor cells aim the restoration of DNA damage rather than further genomic derangement. 4. In tumors, estrogen signal drives the pathways of DNA stabilization leading to apoptotic death. 5. In the peritumoral cellular infiltration, the genomic damage of tumor induces inflammatory cytokine secretion and increased estrogen synthesis. In the inflammatory cells, increased growth factor receptor (GFR) signal confers unliganded activation of estrogen receptors (ERs). 6. In tumor cells responsive to genotoxic therapy, constitutive mutations help the upregulation of estrogen signal and consequential apoptosis. In tumors non responsive to genotoxic therapy, the possibilities for ER activation via either liganded or unliganded pathway, are exhausted leading to farther genomic instability and unrestrained proliferation.
Conclusion Understanding the real character and behavior of human tumors at molecular level suggests that we should learn the genome repairing methods of tumors and follow them by supportive therapy rather than provoking additional genomic damages.
Keywords:
Subject: Medicine and Pharmacology - Oncology and Oncogenics
1. Introduction
Cancer is a complex disease presumably originating from mutations in genes promoting genomic instability and initiating cancer development [1]. In tumors, mutagenesis drives DNA damage response (DDR) via altered regulatory pathways increasing genomic instability and helping proliferative activity [2]. In tumors, altered DNA damage response serve the maintenance of survival and unrestrained proliferative activity of cells. These considerations led to the standard therapeutic strategy against cancer: the disruption of mutation activated DNA repair pathways of tumors should lead to clinical recovery of cancer patients [3]. However, the derangement of mutation driven DNA repair technique of tumors could not bridge the gap between basic research and clinical practice.
In tumors, accumulation of somatic mutations yields so called cancer driver genes and their altered regulatory protein products may manage the aggressive expansion [4]. Catalogues of genes known to be involved in cancer development were prepared by whole-exome and later whole-genome sequencing of numerous tumor samples. Analyses of thousands of cancer genomes return to a remarkably similar catalogue of around 300 genes that are mutated in at least one cancer type. Yet, many features of these mutated genes and their exact role in cancer development remain unclear. Accumulation of certain mutated genes in tumors is not enough to justify their pro-oncogenic nature.
There is a close collaboration between the activity of immune system and cancer driver mutations. The immune system has a strong impact on determining the expression of certain cancer driver genes [5]. At the same time, the appearance of certain cancer driver mutations shows correlations with the density and composition of immune competent cells in the tumor microenvironment [6]. The connection of the immune system with the appearance of cancer driver mutations is probably mediated by the fact that all somatic mutations can create neoantigens. These unknown peptides may trigger an immune response eliminating the cell that carries them; this process is known as immune-editing [5].
Cancer driver mutations influence the quantity and composition of immune cell infiltrate in the tumor microenvironment [6,7]. Somatic mutations in cancer driver genes with well known roles in immune signaling, such as CASP8 or HLA, are generally recruiting higher concentrations of immune cells into tumor microenvironment. Most likely, these pro-oncogenic mutations result in immune-evading mechanisms. By contrast, colorectal tumors with accumulated KRAS mutation, show weaker immune cell infiltrate than those without this mutation and the tumors are resistant to immune-checkpoint blockade [8].
Surprisingly, cancer driver genes are exposed even in various healthy cells exhibiting the same somatic mutations like tumors. Two studies examined somatic mutations in the entire human body [9,10]. In some individuals, cancer driver somatic mutations were found in virtually all tissues, although none of them had been diagnosed with cancer. The most interesting recent finding is the presence of somatic PTEN, KMT2D, and ARID1A mutations in healthy liver cells [11]. Hepatocytes showing these well known cancer driver mutations exhibited conspicuously increased fitness, faster expansion and regeneration under stress or other injury as compared with their counterparts without mutation.
The study on liver cells showing high fitness and regenerative capacity despite their cancer driving mutation justifies the positive impact of somatic mutations on genomic stability rather than tumor promotion. There is a plausible explanation; concentration of genome driver somatic mutations in tumors is not a pro-oncogenic effort but rather DNA stabilizer action via genomic plasticity.
Molecular cancer therapies targeting the altered DNA damage response pathways lead to continuous failures. The problem evokes that some modern cancer therapies might cause more harm than benefit as we do not exactly understand the molecular events in the background of diseases [12]. Analysis of therapeutic failures urges a complete turn in our anticancer strategy rather than farther developing and improving the families of moderately effective or even genotoxic drugs.
The aim of the present study is to justify that tumor cells are not enemies to be killed but rather ill human cells having the remnants of the same regulatory pathways like patients’ healthy cells [Compensatory]. Understanding the real character and behavior of human tumors at molecular level suggests that we should learn by watching the genome repairing methods of tumors instead of provoking additional genomic damages.
2. Use of Endocrine Disruptor Synthetic Estrogens in Human Therapy Mistakenly Strengthened the Concept of Estrogen Induced Breast Cancer
In the early 1940s, synthetic estrogens were developed for medical purposes; for the treatment of miscarriage and menopausal complaints and later for oral contraception. Diethylstilbestrol (DES) was a non steroidal hormone; ethinylestradiol (EE) was a steroidal product, while conjugated equine estrogens (CEEs) were extracted from biological samples [14].
Increased breast cancer risk in DES treated patients mistakenly suggested that synthetic estrogens activate the same subcellular pathways like high endogenous estradiol level does leading to alterations in all cellular functions, including interactions with DNA [15]. In reality, malformations and increased breast cancer risk induced by prenatal exposure to DES may be attributed to deregulation of estrogen signaling pathways. In animal experiments, DES and EE treatment provoked histone modification and further genomic damages via ER deregulation justifying their endocrine disruptor character [16].
The development of synthetic estrogens, including both DES and EE, may be regarded as pharmaceutical mistake as they are endocrine disruptors. Endocrine disruptors exhibit a special toxicological mechanism; higher doses induce more genomic damages as compared with lower doses; however, there are no safety low levels of these chemicals [17]. Low doses of synthetic estrogens exert an inhibitory effect on the ligand independent, ancient AF1 domain of ERs, while inducing compensatory estrogen-like activation on the ligand dependent AF2 domain. Conversely, high doses of synthetic estrogens provoke a serious imbalance between the liganded and unliganded activation of ERs resulting in uncompensated damages in the whole genomic machinery [18].
2.1. Synthetic Hormone Use Causes Controversial Correlations between Menopausal Hormone Therapy (MHT) and Women’s Health
For menopausal hormone therapy (MHT), both synthetic EE and CEE extracted from biological samples were prescribed [19]. From the 1940s, MHT became widely used among postmenopausal women for the treatment of menopausal symptoms and prevention of chronic illnesses, such as cardiovascular and thromboembolic complications and osteoporosis. Among postmenopausal women, the use of estrogens with different origin and even their combinations with synthetic progestins resulted in quite controversial clinical experiences concerning the risks and benefits for arterial and venous thromboembolism and female cancers, especially for the breasts. According to the guidance of Food and Drug Administration (FDA), the benefits of MHT use surpass their risks, while no comparative informations regarding the efficacy and toxicity of bioidentical versus conventional hormones could be found [19].
In the early 2000s, two great Women’s Health Initiative (WHI) studies reported quite controversial results in women underwent to MHT. In 2002, increased risks for breast cancer, thromboembolism and cardiovascular diseases were reported in menopausal women treated with conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) [20]. Conversely, in 2004, another great WHI study reported on a striking reduction of breast cancer risk in women treated with CEE (Premarin, Pfizer) alone [21]. The protective effect of Premarin with natural origin may be explained by the omission of the highly toxic progestin, MPA [22].
In 2019, a great meta-analysis study reported worldwide epidemiological evidences of the breast cancer inducing capacity of MHT independent of the used hormone formulas and timing of treatment [23]. All MHT studies reporting the breast cancer preventive effect of Premarin alone were omitted from this analysis. The concept of estrogen induced cancer was both the starting point and the goal of investigation creating a circular reasoning.
In 2020, the earlier WHI study was repeated on the survivor women eighteen years following the MHT and the results reflected long lasting breast cancer preventive effect of Premarin. Both morbidity and breast cancer associated mortality were significantly decreased among estrogen treated women [24]. These results justified long term genome stabilizer power of natural estrogen treatment without synthetic progestin use [18].
In 2021, Premarin treatment of women with ER positive, PR negative breast cancers (N=10739) resulted in significant reduction of tumors and breast cancer related deaths. Authors established, here is the time for change in their breast cancer risk reduction strategies in clinical practice [25].
Analysis of the results of MHT studies using different hormone schedules justified that horse urine derived Premarin without synthetic progestin is a highly beneficial formula against breast cancer, coronary heart disease, thromboembolism, and bone loss [22]. Although, only synthetic hormones may be blamed for breast cancer development and further complications in MHT user women, the breast cancer inducing capacity of endogenous estrogens remained evidence based fact.
2.2. Oral Contraceptives are Endocrine Disruptors Causing either Increased or Decreased Cancer Risk in Different Organs Depending on Their Regulatory Features
Oral contraceptives (OCs) comprising synthetic EE were developed in the 1960s. OCs may induce serious toxic side effects, such as venous thromboembolism, stroke and cardiovascular diseases [Lidegaard 53]. OC use induced deregulation of ER signal and led to an increased risk for insulin resistance and metabolic diseases [26].
Wide spread use of OC use among premenopausal women caused highly ambiguous correlations with cancer risk at different sites. Among OC user women, a slightly increased risk for overall breast cancer was observed [27], while strongly increased risks for ER/PR negative and triple-negative breast cancer (TNBC) were registered [28,29]. Conversely, OC use significantly reduced the risk of endometrial [30], ovarian [31] and colon cancer risk [32]. The controversial correlations between OC use and reduced or enhanced cancer risk at different sites strongly justified that ethinylestradiol is an endocrine disruptor compound rather than a bioidentical estrogen [18].
In BRCA gene mutation carriers, long term OC use significantly increases the risk for overall breast cancer as compared with non carriers [33]. Long term OC use in BRCA mutation carriers, may exert an additional inhibition on the non liganded ER activation aggravating mutation associated weakness of ERs. Conversely, in women, with BRCA1/2 gene mutations, the risk for ovarian cancer is strongly reduced by OC use [34] via exerting an advantageous estrogen-like effect by indirect activation of the AF2 domain [18].
Despite the known metabolic, thrombotic and carcinogenic complications of OCs, they are widely used in medical practice. Clinicians do not believe or do not want to believe the endocrine disruptor nature of OCs. In addition, OC use strengthened the misbelief that endogenous estrogens in higher concentrations may induce increased breast cancer risk.
3. In BRCA Gene Mutation Carrier Cells, the Defect of Liganded ER Activation is the Initiator of DNA Damage and Cancer Development
BRCA gene mutation carrier patients are pathological models for genomic instability and increased predisposition for breast and ovarian cancer development. The first breast cancer gene (BRCA1) was identified in 1994 showing close correlation with breast cancer development when becoming mutated [35], while the second breast cancer gene (BRCA2) was announced in 1995 [36]. BRCA1 and BRCA2 genes may be regarded as safeguards of the genome. Their BRCA protein products control DNA replication, transcriptional processes, DNA recombination and the repair of DNA damages [37].
Although functional BRCA proteins have crucial role in the health of all cell types in men and women, BRCA gene mutations are preferentially associated with tumor development in female breasts and ovaries [38,39].
The tissue specificity of BRCA1 mutation associated tumors suggested a potential relationship between BRCA1-loss and excessive estrogen signaling in breast cancer development. However, BRCA1 mutation linked tumors are typically ER-alpha negative, poorly differentiated and show rapid growth and poor prognosis [40]. Receptor expression profiling of BRCA1 mutant tumors showed that their vast majority proved to be ER-alpha negative and ER/PR/HER2 negative, nominated as triple negative breast cancer (TNBC) [41]. In addition, the development of ER-alpha negative breast cancer has been reported to be a predictor of BRCA1 mutation status in patients [42]. In sporadic ER-alpha negative breast cancers, reduced BRCA1 expression and decreased level of ER-alpha mRNA were parallel observed, while estrogen treatment increased BRCA1/2 mRNA levels [43]. These results suggest that BRCA gene mutation deteriorates the regulatory interplay with ERs leading to decreased ER expression and consequential decreased estrogen signal [44].
Since the regulation of female breast requires strict balance between liganded and unliganded ER activation, the weakness in ER expression and estrogen activation results in preferential susceptibility to genomic damage in the breasts of BRCA mutation carrier women [44]. In diabetes and obesity, weak estrogen signal associated defects of hormonal and metabolic equilibrium are directly associated with increased TNBC risk.
Molecular studies on interactions between BRCA1 protein and ER alpha yielded highly controversial results supporting either upregulating or downregulating effect of BRCA1 on ER alpha transactivation.
Wild type BRCA1 gene was demonstrated to inhibit ER alpha transcriptional activity under the control of its estrogen responsive elements [45]. BRCA1 could suppress the expression of near all estrogen regulated genes [46]. In addition, BRCA1 was able to inhibit p300 mediated ER acetylation, which is essential for the transactivation of ERs [47]. By contrast, it was reported that BRCA1 may induce an increased transcriptional activity of ER alpha by upregulation of p300 expression, a coactivator of ER alpha [48]. Similarly, BRCA1 ensured coactivator Cyclin D binding to ER alpha so as to facilitate the transcriptional activity [49].
The controversial findings reflect the complexity of regulatory processes including both activation and repression. In conclusion, estrogen liganded ER alpha may choose momentarily appropriate cofactors, promoter regions and transcriptional pathways in harmony with optimal BRCA1 expression and activation [50].
In genome stabilization, BRCA and ER proteins are in mutual interaction by direct binding regulating each other’s activation [51]. The amino-terminus of BRCA1 increases the activation of ER alpha, while the carboxyl-terminus of BRCA1 may function as a transcriptional repressor on ER alpha protein. ER alpha and BRCA1 are crucial components of the regulatory circuit of DNA stabilization as well [50]. Defective expression or activation of either BRCA1 or ER alpha protein disturbs their interaction, endangering both estrogen signal and genomic stability.
In women with BRCA gene mutation, anovulatory infertility frequently occurs [52] reflecting the defects of liganded estrogen signal. In addition, early menopause associated with ovarian failure is characteristic finding in BRCA mutation carriers [53]. In 85% of BRCA1 mutation carriers, loss of functional BRCA1 protein correlated with elevated aromatase levels and increased estrogen synthesis [54] suggesting compensatory actions against decreased ER expression.
In BRCA mutation carrier breast cells, decreased BRCA1 protein synthesis is associated with down-regulation of ER alpha mRNA expression and low ER alpha expression [55]. In BRCA gene mutation carrier tumor cells, a consequently decreased liganded activation of ERs was observed [45]. In BRCA gene mutation carrier breast cancer cells a decreased expression of ER alpha was experienced [56].
The defect of liganded ER activation in BRCA mutation carriers is a crucial finding as it explains the increased inclination to cancers, the ER negativity of developing tumors and the ovulatory disorders of female patients.
In BRCA Mutation Carriers, Both Healthy Cells and Tumor Cells Show Compensatory Molecular Changes Improving the Decreased Estrogen Signal
In BRCA mutation carrier women, the defect of estrogen signaling endangers the genome stability in healthy cells, and means a risk for further genomic deregulation for tumor cells. In healthy cells with BRCA mutation, a compensatory upregulation of estrogen signal may preserve genomic stability, while in BRCA mutation carrier tumor cells increased estrogen signal may protect from further genomic damage and increasing proliferative activity. Tumor cells possess the remnants of the same genome stabilizer pathways like healthy cells have. In the emergency situation of weakening estrogen signal, tumor cells may show various activating mutations increasing both liganded and unliganded ER activation [57].
Healthy cells. In mammary epithelial cells, loss of BRCA1 gene leads to increased epidermal growth factor receptor expression [58], which means an unliganded activation of ERs instead of pro-oncogenic impact. In BRCA1 mutation carrier women, BRCA1 protein activity confers the selection of appropriate CYP19 aromatase promoter region for the compensatory intensifying of estrogen synthesis [59]. In mammary fibrous adipose cells, downregulation of BRCA1 gene increased the specific activation of the PII promoter on Cyp19 aromatase gene leading to increased estrogen synthesis. Mutation of BRCA1 gene may be counteracted by unliganded activation of ERs conferred by upregulation of growth factor receptors and P13K/Akt pathways via interaction with BRCA1 protein [60].
Tumor cells. In BRCA1-deficient human ovarian cancer cells, ER alpha exhibited increased ligand independent transcriptional activity that was not observed in BRCA1 proficient cells [61]. Authors suggested that loss of BRCA1 increased unliganded ER activation; however, it was a compensatory activation attributed to the defective liganded activation
In tumor cell line with BRCA mutation, increased estrogen signal was observed via enhanced activation of p300, a transcriptional coactivator of ERs [48]. In familiar breast cancers with BRCA mutation a further transcriptional activator of ERs, Cyclin D1 was highly accumulated [62]. Nuclear factor kappaB (NF-κB), an important ER coactivator, was persistently activated in a subset of BRCA1-deficient mammary luminal progenitor cells [63].
In sporadic breast cancer cells, wild BRCA gene is capable of increasing the expression of the coding gene of ER alpha, ESR1, mediated by the activator Oct-1 [56]. Moreover, BRCA could transcriptionally increase the expression of ER alpha mRNA.
Studies on BRCA mutation carriers teach us crucial new aspects for cancer research. 1. Genome instability is linked with the weakness of liganded ER activation rather than with excessive estrogen signal. 2. BRCA gene mutation carrier healthy cells are working on the improvement of endangered DNA, via upregulation of both liganded and unliganded ER activation. 3. In BRCA mutant tumor cells, the upregulation of estrogen synthesis and unliganded ER activation are efforts protecting DNA from further damage. 4. Both healthy and tumor cells with BRCA gene mutation, exhibit gene amplification and activating gene mutations so as to increase estrogen synthesis and to improve ER activation 5. In BRCA mutation carriers, the whole body works on genome stabilization via increased ovarian and peripheral estrogen synthesis.
4. Estrogens are Principal Regulators of Genomic Machinery in Mammalian Cells
At cellular level, estrogen activated ERs (ER alpha and ER beta) are the hubs of genomic machinery orchestrating all cellular functions affecting both somatic and reproductive health [64]. Molecular players of all cellular mechanisms are recruited into regulatory circuits receiving their commands directly or indirectly from ERs and in turn, sending their signaling reports back to ERs.
DNA stabilizer circuit regulated by ER-alpha. Estrogen activated ER-alphas are the primary initiators and organizers of the regulatory circuit for DNA stabilization in a triangular partnership with genome safeguarding proteins, such as BRCA1 and aromatase enzyme (A450). The promoter regions of ESR1, BRCA1, and CYP19 aromatase genes exhibit a strong interplay for the appropriate expression of ER-alpha, BRCA1 protein and aromatase enzyme [50]. Upregulation of ER signaling is the safeguard of DNA stability in both amplifying and quenching phases of cell proliferation..
Activated ER-alpha as a transcriptional factor drives ESR1 gene expression inducing expression of protein coding ER-alpha-mRNA and ER-alpha protein. Activated ER-alphas also have the capacity to occupy BRCA1 promoter regions increasing the expression of protein-coding BRCA1 mRNA transcripts and elevated BRCA1 protein synthesis [38].
BRCA1 protein as a transcriptional factor induces the transcriptional activity of BRCA1 gene and increases BRCA1 protein expression. BRCA1 protein activates the expression of ESR1 gene and a consequential increased ER-alpha synthesis [56]. In addition, BRCA protein may occupy the CYP19A promoter region, which is BRCA1 responsive and confers an increased expression of aromatase enzyme. BRCA1 protein ensures a safety balance between the expression of ER-alpha protein and aromatase enzyme [57].
Abundant BRCA1 proteins may induce epigenetic modification and activating mutations on ESR1, BRCA1 and CYP19 aromatase genes via increasing appropriate lncRNA expression and resulting in increased production of the three regulatory proteins: ER, BRCA1 and aromatase [57]. In addition, abundant BRCA1 protein upregulates the transcriptional activity of ER-alpha conferred by either Cyclin D1 [49] or p300 coactivator protein [48]. Increased BRCA1 activity mediates a repressed unliganded activation of ERs [61], while a compensatory increase in liganded ER-activation improves DNA stability [18]. Further lncRNA transcripts of BRCA1 may stimulate amplification on CYP19 aromatase promoter gene increasing A450 aromatase enzyme expression and estrogen synthesis [59]. Increased estrogen concentrations bind and activate abundant ER-alphas, further stimulating the circuit of DNA stabilization [50].
BRCA1 and ER-alpha proteins are capable of direct binding as well; as transcriptional factors. Certain binding sites drive upregulative processes, while others may silence each other’s transcriptional activity [51]. Mutagenic alteration or low expression of ER-alpha may dangerously decrease the expression of BRCA1 mRNA transcripts and BRCA1-protein synthesis; weakening DNA-safeguarding [43]. In turn, decreased or defective synthesis of BRCA1-protein leads to downregulation of both ER-alpha mRNA expression and ER-alpha protein synthesis [55]. Loss or defect in either ER-alpha or BRCA1 protein function results in genome instability and increased cancer risk [50].
Cell proliferation circuit regulated by ER-alpha. The main regulator of cell proliferation is the estrogen activated ER-alpha in strong interplay with membrane associated tyrosine kinase growth factors receptors; epidermal growth factor receptor (EGFR), and insulin-like growth factor receptor 1 (IGF-1R) [13]. Balanced liganded and unliganded ER-alpha activation ensures a strong control over DNA replication during both increased and decreased cell proliferation. Collaboration between ER and GFR receptor families for the regulation of cell growth and proliferation may be more or less maintained even in malignant tumors [18]
IGF-1R exhibits a bidirectional signaling pathway with estrogen activated ERs [65]. IGF-I expression is regulated by insulin and growth hormone (GH), which stimulate the synthesis of IGF-I in the liver [66]. IGF-1 binding to IGF-1R activates two main signaling pathways: the phosphatidylo-inositol 3-kinase (PI3K)-AKT) and the Ras-mitogen-activated protein kinase (MAPK) pathways. These kinase cascades stimulate an unliganded transcriptional activity of ER-alpha via phosphorylation of serine residues [67].
ERs are capable of stimulating many proteins in the insulin-IGF-1 system, including IGF-1R and insulin receptor substrate 1 (IRS-1) [68,69]. ER-alpha binds and phosphorylates IGF-1R and controls its signaling pathways. In IGF-1 knock-out mice, estradiol induced uterine growth is missing [70]. In turn, in vivo IGF-1 activation of uterine cell proliferation is strongly dependent on ER-alpha activity [71].
Estrogen treatment stimulates the synthesis of EGF in uterine epithelium via ER-activation leading to proliferative effect [72]. In the absence of estrogen, EGFR signaling may be activated via unliganded ER activation [73]. Conversely, in the uterus of ER-alpha knock-out mice, EGF induced DNA synthesis and transcription was completely missing [74]. In ovariectomized mice, 17-beta-estradiol treatment caused a rapid transient upregulation of uterine EGFR mRNA and protein levels and increased the number of EGF-binding sites through ER activation [75].
In the nucleus, EGFR signal is capable of phosphorylation and activation of ER-alpha at serine 118 through the growth factor receptor activated MAPK pathway [76,77]. Phosphorylation at serine 118 increases ER-related transactivation of several genes that are upregulated by EGFR. Growth factor receptor signal may also increase the transcriptional activity of nuclear ERs via the phosphorylation of their coactivator proteins, including steroid receptor coactivator 1, p300 protein and cyclin D1 [78,79].
Cytoplasmic, estrogen-activated ERs induce an upregulation of PI3K signaling pathway via EGFR activation [80]. In endothelial cells, PI3K activation by estrogen treatment led to a rapid upregulation of 250 estrogen regulated genes within 40 minutes [81]. The ER/EGFR cross-talk at the membrane ensures the activation of multiple signaling pathways that further increases the extensive transcriptional activity of ERs [65].
In human breast cancer, the expression of ERs and EGFRs exhibit an inverse correlation [82,83]. In tamoxifen responsive breast cancer cell lines, a compensatory increased expression of ERs may be observed improving estrogen signal. In tamoxifen resistant tumors, an additional high expression of growth factor receptors may be observed [84] increasing the unliganded activation of ERs. Abundant GFRs extremely increase the unliganded activation of ERs; however, they cannot counteract the artificial blockade of AF2 domain [18].
Fuel supply circuit regulated by estrogen activated ER-alpha. Estrogen activated ER-alpha drives a regulatory circuit for the maintenance of glucose homeostasis and upregulates all steps of cellular glucose uptake providing fuel for all cellular functions [50]. Defects of eatrogen signaling lead to serious difficulties in cellular glucose uptake designated as insulin resistance and result in serious chronic diseases including cancer [85]. In conclusion, insulin resistance is the link between defective estrogen signal and increased cancer risk.
Estrogen regulated genes stimulate insulin secretion, insulin receptor expression and activation [86]. When insulin binds to insulin receptor, auto-phosphorylation of multiple tyrosines initiates the activation of insulin signal transduction [87]. Activated ERs may upregulate the expression and functional activity of intracellular glucose transporter-4 (GLUT4) facilitating insulin assisted glucose uptake [88]. ER-alpha regulates the insulin receptor substrate 1 (IRS1) mediated activation of PI3-K/mTOR signaling pathway that closes the regulatory circuit by unliganded activation of nuclear ERs [89].
Estrogen signal facilitates glucose uptake even in cancer cells providing energy for the self directed restoration of DNA stability. In MCF-7 human breast cancer cell line, estradiol increases the expression of insulin receptor substrate-1 (IRS-1) potentiating insulin signaling [90]. In ZR-75-1 breast cancer cells, estrogen/progesterone treatment upregulated glucose transporter 1 (GLUT1) expression [91]. In MCF-7 cell lines, estradiol treatment activated ERs upregulating PI3K/Akt signaling pathway parallel with a facilitated translocation of glucose transporter 4 (GLUT4) vesicles to the plasma membrane [92]. Defective or inhibited estrogen signal leads to failure of glucose uptake even in tumor cells, decreasing the possibilities for genome stabilizer processes.
5. Estrogens Are Master Regulators of Metabolism and Energy Homeostasis in the Whole Body via Orchestrating Adipose Tissue Functions
Adipose tissue deposited all over the body, provides energy and epigenetic regulatory commands for all tissues and organs via its estrogen activated ER network. In healthy adipose tissue, estrogen signal regulates the glucose homeostasis and the balance of lipolysis/lipogenesis [93,94]. In adipose tissue, damaged estrogen signal leads to defect of all regulatory functions and serious diseases may develop in the fat regulated visceral organs, cardiovascular structures and hemopoietic bone marrow [95].
The subcutaneously located adipose tissue provides energy and estrogen regulation for the skin and the skeletal muscles. Centrally positioned fatty tissue within the trunk and abdomen closely surrounds the visceral organs and cardiovascular structures, [96]. Visceral fat is largely located in the omental and mesenteric adipose tissue in the vicinity of stomach, intestines, liver and pancreas. Kidneys and the attached adrenal glands are embedded into abundant fatty tissue capsule. Adipose tissue deposition within the visceral pericardium surrounds the myocardium and coronary arteries providing estrogen signal and energy for the moving heart. Perivascular adipose tissue is nursing most blood vessels with the exception of pulmonary and cerebral arteries [97]. Further depot of adipose tissue is gonadal fat (GAT) surrounding the ovaries and testes having specific regulatory functions [98].
Female breasts enjoy exceptional nursing level as mammary lobules are intimately intermingled with the estrogen and ER rich fatty tissue pad [99]. This close connection between adipocytes and mammary cells is associated with the extreme demand of breasts for strict regulatory control and abundant energy supply. This high regulatory claim of the breasts may explain their unique vulnerability to estrogen loss or defect of ER activation.
The third largest fat depot is the bone marrow fat following subcutaneous and visceral fatty tissue. Adipocytes are active components of the bone marrow microenvironment regulating hemopoietic and immune cell proliferation and function via their estrogen signal and secretome [100].
Interestingly, the central nervous system does not enjoy the estrogen driven adipose tissue safeguard, while the brain shows extreme claim for estrogen regulation. Recently, microbial sequences were found in healthy human brain samples [101] suggesting that they may provide important support for cerebral functions. Microbiom in the gut has great role in increasing unbound, free estrogen levels via their β-glucuronidase activity [102,103]. It is a plausible possibility that gut microbiom colonized in the brain increases the level of accessible, free estrogen.
Adipose tissue is a major source of estrogen synthesis among extragonadal sites in both women and men [104]. Circulating and locally synthesized estrogen hormones regulate the functional activity of adipose tissue. Estrogens synthesized in adipose tissue are acting locally, in an autocrine manner, while increase ER activation in the adjacent organs in a paracrine manner [105]. Estrogen hormone is the main regulator of adipose tissue health via metabolic and epigenetic pathways [106]. Estrogen exerts its physiological effects on estrogen responsive adipocytes via estrogen receptors (ERα, ERβ and GPR30) [107].
In the gonads, C19 steroids are essential precursors of estrogen synthesis, while extragonadal sites are unable to synthesize estrogen directly from C19 steroids. With ageing, increased estrogen synthesis in adipose tissue requires precursor supply, such as dehydroepiandrosterone (DHEA) from external sources [108].
The noteworthy volume of ubiquitous adipose tissue and its remarkable estrogen synthesis justify that adipocytes have crucial roles in controlling and regulating the signaling network of adjacent organs and the whole body.
Secretory Functions of Visceral Adipose Tissue in Healthy Lean and Obese Patients with Defective Estrogen Signal
Abdominal adipose tissue has crucial physiological secretory functions [109]. Adipokines, cytokines and growth factors are important signaling molecules in adipose tissue and their estrogen regulated activation ensures the health of the whole body.
Sexual steroids. In adipose tissue, estrogen synthesis and appropriate estrogen signaling regulates the expression of numerous genes and the harmonized synthesis of signaling molecules [106].
Adipokines. Leptin regulates the energy balance in the hypothalamus exerting anorexinogenic and lipolytic effects. Estrogen treatment increases the expression of leptin receptors amplifying the leptin-sensitivity of various cells [110]. In estrogen deficient aromatase knock out (ARKO) mice, visceral adiposity develops and leptin levels are highly elevated [111]. Adiponectin is protective against insulin resistance mitigating various inflammatory reactions, and improving endothelial functions. Oophorectomy increases adiponectin levels in adult mice, while it may be reversed by estradiol substitution [112]. Resistin level increases parallel with obesity, which may be a compensatory reaction. In subcutaneous adipocytes, an estradiol benzoate injection decreases resistin levels [113].
Proinflammatory cytokines and low grade inflammation. Proinflammatory cytokines are regulatory proteins having great role in the maintenance of genomic and metabolic stability. In obese fatty tissue, low grade inflammatory reactions and abundantly expressed cytokines are counteractions to genomic deregulation via increasing estrogen signaling [114]. Insulin resistance of obese estrogen deficient adipose tissue leads to further regulatory disorders in the adjacent organs resulting in serious co-morbidities, such as fatty degeneration and malignancies [115,116].
In the low-grade inflammation of obese adipose tissue, increased levels of inflammatory cytokines and immune cell infiltration comprising macrophages and T cells may be found [117]. Proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) generate an increased expression and activation of aromatase enzyme resulting in increased estrogen synthesis [118]. Proinflammatory cytokines have beneficial effects against obesity and obesity related metabolic disorders via increasing aromatase activity and estrogen synthesis. Estrogen treatment of obese ovariectomized mice decreased the expression of inflammatory cytokines, included TNFα and the upregulation of estrogen signal improved the insulin sensitivity in both adipose tissue and liver [119].
Insulin-IGF system. The insulin like growth factor (IGF) system is involved in regulation and control of physiologic growth and differentiation. Insulin and insulin-like growth factor receptors act as ligand-specific modulators regulating genes on similar pathway [120]. In the early phases of insulin resistance, a compensatory increased IGF-1 level mediates increased insulin synthesis resulting in compensatory hyperinsulinemia.
Strong crosstalk and interplay between signaling pathways of ERs and growth factor receptors (IGF-1R, EGFR, VGFR) are well known in both health and disease [121,122]. Among physiological circumstances, growth factor activated ERs are capable of either stimulating or silencing cell growth and proliferation. In deregulated tumor cells, growth factor activated unliganded ERs do not turn to excessive proliferative stimulus, but rather they are initiators of DNA stabilization and apoptotic death.
Estrogens regulate both insulin like growth factor 1 (IGF-1) synthesis and the expression of its receptor (IGF-1R) in adipocytes. In turn, an increased synthesis of IGF-1 and its receptor upregulates the AKT and MAPK pathways promoting an increased unliganded activation of ERs [123]. In an estrogen deficient milieu, unliganded activation of ERs via IGF-1 receptor signaling may transiently ensure the genome wide expression of estrogen regulated genes [64]. In conclusion, in obesity and insulin resistance, increased expression and activity of IGF-1 receptors are not pro-oncogenic pathways but rather they increase unliganded ER activation.
Interaction between adipocytes and immune cells. Adipocytes are in signaling crosstalk with immune cells in both healthy and obese adipose tissue. In lean adipose tissue, IL-4 secreted by eosinophil granulocytes and regulatory T (Treg) cells activate M2 type macrophages, which express arginase and anti-inflammatory cytokines such as IL-10. In contrast, in obese adipose tissue, high number of M1 type macrophages and increased secretion of pro-inflammatory cytokines, such as TNFα and IL-6 are coupled with a decrease in anti-inflammatory immune cells [117]. In animal experiments, estrogens are capable of improving metabolic disorders and at the same time they exert anti-inflammatory effects. In female mice, estrogen protects from adipocyte hypertrophy, obesity and prevents adipose tissue oxidative stress and inflammation [124].
In obesity, upregulation of estrogen signaling restores insulin sensitivity, reduces lipid deposition, decreases pro-inflammatory cytokine synthesis and quenches inflammatory infiltration. Estrogen treatment provides quite new ways for the prevention and cure of obesity and obesity related complications.
6. Tumor Cell Itself Is the Frontline of Anticancer Combat via Increasing Estrogen Signal and Expression of Estrogen Regulated Genes
According to global medical concepts, tumor cells are enemies to be killed as they presumably fight for their survival similarly like pathogenic bacteria fight against antibiotics. Seemingly, tumor cells express cancer driver genes via somatic mutation and their altered protein products defeat both the immune defense of body and the therapeutic effect of pharmaceutical agents.
In reality, the recognition of DNA damage means an emergency state even for tumor cells. Upregulation of estrogen signal via liganded and/or unliganded pathway is the appropriate means for the restoration of DNA stability. However, in tumors, the possibility for DNA repair is questionable attributed to the genomic damage. The more differentiated a tumor, the stronger is its capacity for compensatory upregulation of estrogen signal coupled with DNA restorative efforts [125].
Spontaneous healing of early breast tumors is a well known finding justifying the capacity of initial cancers for self directed remission. A systematic review and meta-analysis study evaluated high prevalence of incidental breast cancer and precursor lesions in autopsy studies on clinically tumor-free cases. The estimated mean prevalences of incidental cancer and precursor lesion were surprisingly high: 19·5% and 0·85% [126].
Breast cancer is regarded as a multifactorial and very heterogeneous disease that refers to the abnormal proliferation of the lobular and ductal epithelium of the breast resulting in tumor formation [127].The classifications of breast cancers follow the recommendations of the World Health Organization (WHO), which are regularly revised in accordance with the scientific progress [128].
The most important parameter for the classification of breast cancers is their molecular profile, as it was described in 2000 [129]. The heterogeneity of breast cancers at molecular level was revealed through the various expression of a panel of genes. Breast cancers were divided into four main groups: 1. luminal A (60% of cases), 2. luminal B (10% of cases), 3. overexpression of human epidermal growth factor receptor 2 (HER2) (20% of cases) and 4. basal-like triple-negative breast cancers (TNBCs) (about 10% of breast cancers). Another subgroup has also been described as a normal breast-like subcategory which resembles the luminal A group but shows a worse prognosis.
In clinical practice, these tumor groups are identified by immunohistochemical markers, such as ER-alpha, progesterone (PR) and human epidermal growth factor receptor (HER2) expression [127]. In breast cancers, the overexpression of certain receptor families is mistakenly regarded as aggressive survival technique and their targeted inhibition is the principle of current therapeutic measures. In reality, missing or decreased expression of certain receptors in tumor cells highlights the points of genomic defects requiring repair. In tumors, overexpression of certain receptors and regulators, as well as activating mutation of their genes indicate the efforts for self directed genomic repair rather than developing survival techniques [13,57]. In reality, the loss of certain receptors indicates the genomic damage, while the overexpression of others represents the genome repairing effort.
Immunohistochemical markers of breast cancers show the alterations in their gene and receptor protein expression as compared with healthy breast epithelium. Molecular alterations reflecting the grade of DNA damage and the concomitant DNA repairing actions in different breast cancer types are shown by Table 1.
Luminal type A cancers are the least aggressive tumors with expression of ER alpha, and PR. Increased ER expression in breast tumors is traditionally regarded as a crucial inducer and promoter of tumor growth [127]. This concept derives from confusing the constellation with causation. Increased ER expression is not a causal factor for tumor growth but rather it is an effort for improving estrogen signal and DNA stabilization in an estrogen deficient milieu [44].
Estrogen receptor expression was shown to be parallel with DNA repair capacity in breast cancer cells [130]. This correlation justifies that high ER expression of untreated tumors is the key to self directed DNA repair, rather than a fuel for tumor growth. The strong belief in estrogen induced cancer does not allow considering opposite alternatives.
Luminal A breast cancer may exhibit transiently good response in 50% of tumors to adjuvant endocrine therapy; however, near all patients, previously showing good tumor responses later become non responders [131]. Patients with early luminal, ER-positive breast cancer are at continuous risk of relapse even after more than 10 years of tamoxifen treatment [132]. These experiences underline that endocrine disruptor therapy is not appropriate method even for early, ER positive breast cancer care.
Luminal B tumors are more aggressive than luminal A types. They express lower ER alpha and lower PR expression or may be PR negative in correlation with the weakening estrogen signal [133]. Luminal B tumors are associated with an increased rate of p53 mutations and in certain B type tumors, HER2 may also be expressed [134]. Activating p53 mutations are not oncogenic changes but rather they mean stronger DNA protection in tumors with weakening genome stability. In luminal B type tumors, appearance of HER2 expression works on the compensatory unliganded activation of ERs [18].
After tamoxifen therapy, patients with ER positive, PR negative and HER2 positive tumors exhibited higher rates of tumor recurrence and mortality as compared with those who did not receive the agent [135]. This observation suggests that in type B tumors, the wakening ER signal is further worsened by endocrine disruptor treatment. In contrast, Premarin treatment of ER positive, PR negative breast cancer cases resulted in significant reduction in tumor size and improved patients’ survival [25].
HER-2-enriched breast cancer is ER- and PR-negative and HER-2-positive. HER-2-enriched cancers tend to grow faster than luminal cancers and can have a worse prognosis. ER- and PR negativity in HER-2 enriched breast cancers reflects loss of estrogen signal and strong defects of all genomic processes. HER-2 overexpression in hormone receptor negative tumors is mistakenly regarded as a trigger for tumor proliferation similarly to all other growth factors [127]. By contrast, in the emergency situation of DNA damage, HER2 overexpression is a compensatory effort for the unliganded activation of ERs occurring scarcely in this tumor type [18]. HER-2 protein targeted therapies against HER-2-enriched tumors show similarly ambiguous results like ER inhibitor antiestrogens against ER positive tumors [13].
Triple-negative or basal-like breast cancer is ER-negative, progesterone receptor-negative, and HER-2-negative. Triple-negative breast cancer is more common in people with BRCA1 gene mutation, younger women, and black women. Triple-negative breast cancers are more aggressive than either luminal A or luminal B breast cancers and they are not responsive to endocrine therapy [127].
In triple negative breast cancers (TNBCs), the lack of ER, PR and HER-2 receptors indicate the serious deregulation of the whole genomic machinery. These tumors are poorly differentiated and clinically show rapid growth and spread. In TNBC type tumors, there is no possibility for self directed DNA repair as ERs seem to be absent or hidden and the regulatory pathways for both liganded and non liganded ER activations are unnoticeable [44]. Increased risk for TNBC type tumors in African-American women may be attributed to their excessive pigmentation in a relatively light deficient geographical region. Poor light exposure leads to metabolic and hormonal alterations conferring increased cancer risk [136].
Molecular classification of breast cancer types reflects the fact that in women, stronger estrogen signal may suppress, while a defective estrogen signal liberates breast cancer initiation and growth [44]. In tumor cells, the higher the ER expression the stronger is the apoptotic effect of therapeutic estrogen exposure. In contrast, endocrine disruptor therapies may achieve only transient tumor responses in appropriately ER positive breast cancers. Poorly differentiated ER/PR negative and TNBC type tumors are refractory to antiestrogen therapy attributed to their serious genomic deregulation.
In conclusion, breast cancers are not multifaceted tumors with quite different etiology and pathogenesis. Consequently, they do not need quite different therapies depending on their receptor status. The levels of regulatory defect create a line of variously differentiated tumors between strongly ER positive, highly differentiated and poorly differentiated TNBC type ones. In breast cancer therapy, natural estrogen is a risk free available option for ER positive tumors [25]. Against ER negative and TNBC type, poorly differentiated tumors, Maloney’s mRNA technology would be a promising therapy to be introduced in the near future [125].
7. Peritumoral Microenvironment: The Second Line of Antitumor Battle for Estrogen Regulated Genes
In the early 2000s, the role of tumor microenvironment emerged as being an important player in cancer development, tumor invasion and metastatic spread [137]. Today, cancer is regarded as a complex disease built up from the neoplastic lump and its altered cellular and stromal microenvironment [138,139]. There is a strengthening belief that tumors insidiously influence all players in their microenvironment via dynamic intercellular communication so as to help their invasive growth via escape from defensive immune reactions and anticancer treatment.
The supposed conspiration between tumors and their microenvironment is based on the belief that all signaling molecules and regulatory proteins are taken for pro-oncogenic factors when their expression is highly elevated in tumors and in the adjacent cellular infiltration [139,140,141]. In addition, when important regulatory genes, such as ESR1, are accumulated or mutated in tumors, they are regarded as pro-oncogenic alterations rather than self regulated efforts for the repair of genomic damages [142,143,144,145,146]. According to the reigning preconception, in tumor cells, the upregulation of estrogen signal and its activator pathways are regarded as keys to tumor growth.
In reality, in tumors, upregulation of certain signaling pathways and activating mutations are not pro-oncogenic factors but rather they are efforts for metabolic improvement and genomic stabilization [57]. Unfortunately, advanced tumors have weakened capacities for self directed genomic repair and they ask for help via sending messages to their microenvironment. In turn, peritumoral activated cells send signals and regulatory molecules helping the tumor to achieve DNA repair and to commit apoptosis as a kamikaze action.
Re-evaluation of studies on the biochemical and genomic communication between tumors and activated microenvironmental cells revealed that all signal messages and transported exosomes aim the upregulation of each other’s estrogen signal and improvement of all genomic functions. These activating processes serve the elimination of the tumor rather than helping its proliferation and invasion. In conclusion, the dynamic communication between the tumor and its microenvironment is a marvelous collaboration among molecular players fighting for genomic repair and apoptosis of tumor by means of their genomic plasticity.
Cancer-associated fibroblasts (CAFs) are major components emerging in the tumor microenvironment. Their assembly and activation may be attributed to signals deriving from cancer cells [138]. CAFs are in continuous signal communication with cancer cells and all other cell types in the tumor microenvironment [139]. Distant intercellular communication occurs by spherical extracellular vesicles (EVs) comprising exosomes carrying different molecules, such as proteins, DNAs, non-coding RNAs, miRNAs and mRNAs. Biochemical and genetic cross-talk between cancer cells and CAFs are important observations; however, the presumed cooperation for tumor invasion and metastatic spread is not justified, it is a biased labeling.
Activation of growth factor signaling cascades. In CAFs, the expression of growth factors, such as insulin-like growth factor (IGF-1), fibroblast growth factor FGF-7, FGF-10, HGF and TGF-beta 2 are regarded as pro-tumorigenic factors [147]. In reality, estrogen receptors and growth factor receptors are common regulators of crucial cellular functions including cell growth and apoptosis as well as metabolic processes even in tumors [65].
Transforming growth factor beta (TGF-beta) superfamily is the main inducer of CAF activation and in turn, CAFs secrete large amount of TGF-beta isoforms for improving tumor cell regulation [148]. Tumor cell-derived extracellular vesicles (EVs) may frequently contain growth factor TGF-beta, which is regarded as typical mitogen factor of tumors [149]. Considering the ER activating role of growth factors, tumors send them to CAFs for activation of their estrogen signal. Tumor derived EVs containing certain miRNAs contribute to the enhanced TGF-beta expression in CAFs through the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway [150]. PI3K and AKT/mTOR pathways upregulate ER activation and improve glucose uptake, which are not pro-tumorigenic processes, but rather increase antitumor activity. Cancer cell-derived EVs containing mRNA coding for CXCR-4 and IGF-1R provoke CAFs for growth factor secretion in acute myeloid leukemia [151].
Cytokines secreted by CAFs, macrophages and immune cells are important regulators of inflammatory processes and immune reactions in the tumor microenvironment [152]. Estrogen signal orchestrates the secretion of both pro-inflammatory and anti-inflammatory cytokines according to the momentary requirements. Pro-inflammatory cytokines stimulate aromatase activity, estrogen synthesis and ER expression in the estrogen responsive peritumoral cellular infiltration. When estrogen concentration reaches appropriately high concentration, the accumulation of anti-inflammatory cytokines will quench the inflammatory reaction parallel with the decreasing estrogen level [114].
IL-1β accumulation in hyperplastic lesions activates CAF formation from fibroblasts via NF-κB pathway [153] that is a coactivator of ERs promoting genome stabilization. Proinflammatory cytokines, IL-6 and TNF-α, are capable of aromatase activation leading to increased estrogen concentration and upregulation of estrogen signal [154]. In gastric cancer, tumor sends miRNA containing vesicles to CAFs so as to induce inflammatory cytokine/chemokine secretion through the Janus kinase (JAK)/STAT and NF-κB signaling pathways [155]. In colorectal cancers, constitutive mutation of KRAS increases the activation of EGFR kinase cascades; PI3K-Akt and RAS-RAF-MAPK, whereas increases RAS-GEF signaling pathway, which is related to abundant cytokine production [156]. In Hodgkin lymphoma, CAFs exposed to tumor cell derived EVs show increased proinflammatory cytokine secretion [157]. CAFs activated by tumor EVs, may in turn shed additional EVs that will transfer signaling and regulatory molecules to tumor cells.
Various tumors promote aromatase activity and estradiol synthesis in the peritumoral stroma via promotion of proinflammatory cytokine secretion [158]. In breast cancers, aromatase is abundantly expressed in tumor cells, intratumoral fibrous cells, and neighboring adipocytes justifying their collaboration in promotion of excessive estrogen synthesis [159]. These observations mistakenly support the role of increased estrogen concentration in tumor growth and invasion.
By contrast, a combined genetic and clinical investigation justified the anticancer capacity of increased local estrogen synthesis in tumors and their stroma. In a large prospective study, examination of the surgical breast tumor samples revealed significant correlation between low aromatase level and an increased loco-regional recurrence rate of tumors [160]. This study suggests that missing estrogen synthesis in tumors is associated with worse prognosis in breast cancer cases.
Circulating estradiol may be systemic modulator of CAF secretome as CAFs express steroid receptors [161]. Estradiol regulates the expression of several microRNAs in CAFs deriving from breast cancer [162]. In gastric cancer, estrogens stimulate IL-6 secretion of CAFs promoting signal transducer and activator of transcription (STAT-3) expression [163]. Increased expression of STAT3 in CAFs secretome confers an effort for genome stabilization as STAT3 is a transcription factor having important role in DNA replication.
Few studies evaluated growth factors and cytokines as positive regulators of the genome rather than pro-tumorigenic factors. TGF-beta was considered as a tumor suppressor factor due to its cytostatic effect on cancer cells [164]. IL-11 was known for its capacity to stimulate platelet production in cancer patients with thrombocytopenia [165].
Immune cells in the tumor microenvironment show intense interaction with tumor cells. The interaction between immune cells and other cell types are regulated by cell surface immune checkpoints [138]. Mast cells are recruited near tumors during tumorgenesis and release a variety of cytokines and chemokines [166]. Cytokines and chemokines are crucial regulators of both genomic and immunologic processes and their accumulation is an anticancer effort. Natural killer cells (NK) are cytotoxic and secrete tumor necrosis factor so as to kill tumor cells [167].
Tumor-associated macrophages (TAMs) infiltrate the microenvironment of tumors and are mainly divided into two categories: classically activated macrophages (M1 type) and alternatively activated macrophages (M2 type). Activated M2 type macrophages are blamed for managing immune escape of tumors. The abundance of TAM infiltration in tumors is mechanically linked with poor disease prognosis [168]. TAM activation and accumulation in tumors is not a pro-oncogenic feature, but rather their intense cytokine secretion is helping aromatase activity and increasing estrogen concentration.
Myeloid-derived suppressor cells (MDSC) have apparently immunosuppressive effects; they may block immunotherapy and may play a role in tumor maintenance and progression [169]. MDSCs also accumulate in response to the chronic inflammation and lipid deposition in obesity and contribute to the more rapid progression of cancers in obese individuals. In reality, accumulation of MDSCs is not a causal factor of rapid tumor progression and obesity associated inflammation but rather it seems to be an intense immune defense against metabolic disorder associated tumors.
Tumor infiltrating lymphocytes (TILs) are important participants of the tumor microenvironment [152]. Immune cell infiltrates may exhibit ambiguous properties either promoting or inhibiting tumor progression depending on the features of primary tumor [170]. CD4+ T cell polarization has been identified as a mediator of tumor immune surveillance. T helper 1 (Th1) cell functions are associated with tumor suppression and upregulation of IFNγ and IL-12. T helper 2 (Th2) responses are reliant of IL-4 production and presumably exhibit tumor promoting activity [171,172]. Murine and human studies reported that increased E2 concentration induces increased Th2 responses and upregulate IL-4 secretion [173,174].
A remarkable fact that constellation of strong estrogen signal and increasing tumor growth does not justify causal correlation. A recent study reported increased immune cell infiltrate comprising Th1 T cells, B cells, and cytotoxic T lymphocytes (CTLs) in ER-negative breast tumors as compared with ER-positive cancers [175]. Correlation between ER-negative breast tumors and more intensive immune cell infiltration strongly suggest that poorly differentiated tumors with loss of estrogen signal need stronger immune support for their DNA repair than highly differentiated ER-positive ones.
Gene expression analysis in ER-positive breast cancer patients showed that blocking the liganded ER activation with aromatase inhibitor (letrozole) continuously increased the tumor infiltration with B cell and T helper lymphocyte subsets following treatment initiation [158]. This result justified that letrozole inhibition of estrogen signal in ER-positive tumors induced an emergency state promptly recruiting strong immune cell infiltration.
In conclusion, tumors and their microenvironment are allies in the fight against worsening genomic defects and consequential tumor invasion. The more serious the genomic damage of a tumor, the denser is the peritumoral immune cell infiltration attributed to the emergency state. Invasive tumor spread coupled with intensive peritumoral cellular infiltration may be regarded as a common failure of tumor and peritumoral cells rather than the victory of presumably conspirator partners.
8. Molecular Changes in Tumors Responsive and Non Responsive to Endocrine Therapy Reflect the Successful or Unsuccessful Counteraction to ER Blockade
The traditional belief of estrogen induced breast cancer required the introduction of inhibitors of estrogen signal for breast cancer care. The pharmaceutical industry developed two kinds of antiestrogens for therapeutic purpose: a selective estrogen receptor modulator, tamoxifen and an aromatase inhibitor (AI), letrozole [176]. Since the early 1970s, antiestrogens are commonly used compounds for breast cancer care as adjuvant therapy.
Antiestrogen therapy of patients with breast cancer yielded many difficulties from the onset attributed to the development of so called endocrine resistance of tumors. Antiestrogen treatment could not surpass the “magic” 30% of tumor response rate, similarly to the weaknesses of other endocrine therapies; such as oophorectomy or high dose synthetic estrogen treatment [177]. The majority of overall breast cancers (≥70%) were not responsive to antiestrogen therapy exhibiting stagnation or even a rapid growth. In addition, about a half of the targeted ER-positive tumors showed primary resistance to antiestrogen therapy [131]. Moreover, near all patients showing earlier good tumor responses to endocrine treatment later experienced secondary resistance leading to metastatic disease and fatal outcome [178].
In the past decades, great efforts were exerted for revealing the mechanism of presumed endocrine resistance of ER-positive breast cancers so as to predict responses to adjuvant endocrine therapy in patients. Researchers mistakenly supposed that both responsive and non responsive tumor cells are aggressive enemies developing various techniques fighting for their survival [13].
8.1. Successful Fight of Antiestrogen Responsive Tumors against the Endocrine Disruptor Treatment
In antiestrogen responsive tumors, the principal action against the blockade of AF2 domain is a compensatory restoration and upregulation of liganded ER activation [57].
1.Tamoxifen treatment facilitates the prompt compensatory unliganded activation of ERs via translocation of ER-alphas out of the nucleus to membrane associated EGFRs [179] (Figure 1.). 2. Long term endocrine therapy upregulates the expression of the most studied coactivator of ER-alpha; AIB1 (amplified in breast cancer 1) [180]. Under tamoxifen treatment, another ER coactivator, cyclin D1 increases the activation of both STAT3 and ERs [181]. 3. Tamoxifen extremely activates the transcription factor NFκB and its upregulative crosstalk with ER-alpha [182,183]. 4. Tamoxifen provokes increasing expression of certain microRNAs that bind to mRNAs of ERs and activate the translational processes [184]. 5. Tamoxifen increases the copy number of ESR1 gene coupled with an increased expression and activation of ERs [185,186] (Figure 2). 6. AI treatment induces an acquired amplification of aromatase encoding CYP19A1 gene enhancing both enzyme expression and estrogen synthesis [187]. 7. In antiestrogen treated tumor cells, copious lncRNA transcripts of ERs confer activating mutations for crucial genes participating in the genome stabilizer circuit; such as ESR1, BRCA1 and CYP19A [57].
In breast cancers responsive to antiestrogen treatment, the facilitated regulatory processes promote the compensatory restoration of liganded ER activation and achieve a successful tumor response [188].
8.2. Unsuccessful Fight of Tumors Becoming Non Responsive to Endocrine Disruptor Treatment
When an earlier antiestrogen responsive breast cancer exhausted the possibilities for liganded ER-activation, the upregulation of unliganded ER-activation through growth factor receptor signal remains as an ultimate refuge for DNA stabilization [18]. However, even an extreme increase in unliganded ER activation is incapable of restoring ER signaling when the liganded pathway is completely blocked (Figure 3.).
In tumors non responsive to antiestrogens, there are physiological pathways for increasing unliganded ER-activation. In tamoxifen resistant tumors, an increased expression of ER coactivator HOXB7, enhances kinase domain phosphorylation of both EGFR [189] and HER2 [190] promoting the unliganded activation of ERs. Estrogen receptor coactivator AIB1 and HER2/neu signaling stimulates hormone independent ER activation [191]. In tumor xenografts, both ER and HER2 activations were associated with the compensatory upregulation of MUCIN4 [192]. In endocrine resistant tumors, an increased expression of either EGFRs [193] or IGF-1Rs [194,195] at the plasma membrane, amplifies the unliganded activation of ERs.
In endocrine resistant tumors, acquired mutations may highly increase the compensatory unliganded activation of ERs.
1.ER mediated mutation of ERBB2 gene of growth factor receptor tyrosine kinases increases the expression and activity of growth factor receptors conferring unliganded activation for ERs [191]. 2. In endocrine refractory ER-positive breast cancer, PIK3CA gene is frequently mutated upregulating the components of the PI3K-AKT-mTOR pathway and increasing unliganded ER activation [196]. 3. AI resistant breast cancers frequently exhibit acquired point mutations in the ligand binding domain (LBD) of ESR1 gene conferring a constitutive hormone independent activation of ERs [197]. 4. In antiestrogen resistant tumors, chromosomal rearrangement affecting ESR1 gene is a further mutational mechanism driving an increased unliganded activation of ERs [198]. 5. In tamoxifen-resistant breast cancer cells, activation of PI3K/AKT pathway is associated with the significant upregulation of BARD1 and BRCA1 protein expressions through an increased unliganded activation of ERs [199].
9. Estrogen Treatment Restores Genomic Regulation and Induces Apoptotic Death in Antiestrogen Resistant Tumors
Estrogen treatment of breast cancers resistant to either long term estrogen deprivation (LTED-R) or tamoxifen (TAM-R) triggers an apoptotic death in tumors [200,201]. Considering the strong upregulation of both ER and GFR expressions in breast cancers unresponsive to antiestrogen treatment, estrogen is capable of exerting its physiological anticancer capacity via a balanced liganded and unliganded activation of abundant ERs. In reality, estrogen does not restore the “antiestrogen sensitivity” of unresponsive breast cancer, but rather helps tumor cells to overcome the poisonous medicament.
Important lessons may be drawn from the 50 years of breast cancer therapy with antiestrogens. 1. In tumors, there is no endocrine therapy resistance but rather the possibilities for compensatory ER activation are exhausted. 2. In tumors responsive to antiestrogen therapy, increased ER expression and activation is not a survival technique but rather it is an effort for increasing estrogen signaling. 3. In tumors non responsive to antiestrogen therapy, increased growth factor receptor expression and activation is not a survival technique but rather it is an effort for compensatory unliganded ER activation. 4. Tumors exhaustively treated by aromatase inhibitor, show genomic plasticity exhibiting acquired mutations on the ligand binding domain of ESR1 gene conferring new, hormone-independent activation of modified ERs in the absence of estrogen.
10. Conclusions
From various organs, female breasts exhibit unique sensitivity to genomic instability caused by either germline or acquired gene mutations. This fact may partially explain that breast cancer has become the flagship of cancer research. Although, the old preconception of estrogen induced breast cancer led breast cancer care to a quite erroneous pathway, thorough examination of the controversies between estrogen signal and cancer development yielded valuable progress in overall cancer research.
Correlation between genomic instability and conspicuously increased breast cancer risk in germline BRCA gene mutation carriers revealed that the defect of genome stabilizer circuit is the origin of cancer initiation rather than excessive estrogen signals. Defect of ER, BRCA or aromatase enzyme upsets the triangular partnership of these regulatory proteins leading to weakness of estrogen signaling and genomic instability. BRCA mutation carrier healthy and tumor cells similarly show efforts for increasing the liganded and unliganded ER activation and for compensatory upregulation of another genome safeguarding protein, p53.
Understanding the fight of cancer cells for activation of estrogen signaling together with genome stabilization reveals the secret of various receptor landscapes of breast cancer subtypes. In tumors, the increased expression of hormone receptors reflects efforts for increasing liganded ER activation, while the overexpression of HER2 means trying to increase unliganded ER activation. The blockade of either ERs or HER2s seems to be an erroneous therapeutic concept. Breast cancers are not resistant to genotoxic therapies but rather they exhausted all possibilities for defending the remnants of genomic stability. Progressive genomic instability leads to unrestrained proliferative activity.
Cellular infiltration of the tumor microenvironment is not organic part of tumors. Inflammatory cells are recruited by the tumor itself and the intercellular communication by messages and extracellular vesicles confer asking help. The stronger the genomic deregulation in the tumor, the denser is the adjacent infiltration of activated mesenchymal and immune competent cells. Immune competent cells do not need therapeutic genomic machination as they exactly know their task in the anticancer fight. When tumor invasion is coupled with dense peritumoral infiltration, supportive genome repairing therapy is necessary rather than the disruption of mutation activated DNA repair pathways of tumor.
In conclusion, the improvement of genomic stability may be the new strategy in cancer therapy. Upregulation of estrogen signal leads to strengthened immune response, whilst induces apoptotic death of tumors in a Janus faced manner.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Klapp, V.; Álvarez-Abril, B.; Leuzzi, G.; Kroemer, G.; Ciccia, A.; Galluzzi, L. The DNA Damage Response and Inflammation in Cancer. Cancer Discov 2023, 13, 1521–1545. [Google Scholar] [CrossRef]
- Sinkala, M. Mutational landscape of cancer-driver genes across human cancers. Sci Rep 2023, 13, 12742. [Google Scholar] [CrossRef] [PubMed]
- Porta-Pardo, E.; Valencia, A.; Godzik, A. Understanding oncogenicity of cancer driver genes and mutations in the cancer genomics era. FEBS Lett. 2020, 594, 4233–4246. [Google Scholar] [CrossRef] [PubMed]
- Marty, R.; Kaabinejadian, S.; Rossell, D.; Slifker, M.J.; van de Haar, J.; Engin, H.B.; de Prisco, N.; Ideker, T.; Hildebrand, W.H.; Font-Burgada, J.; et al. MHC-I genotype restricts the oncogenic mutational landscape. Cell 2017, 171, 1272–1283.e15. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Overman, M.J.; Boutin, A.T.; Shang, X.; Zhao, D.; Dey, P.; Li, J.; Wang, G.; Lan, Z.; Li, J.; et al. KRAS IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019, 35, 559–572.e7. [Google Scholar] [CrossRef]
- García-Nieto, P.E.; Morrison, A.J.; Fraser, H.B. The somatic mutation landscape of the human body. Genome Biol 2019, 20, 298. [Google Scholar] [CrossRef]
- Yizhak, K.; Aguet, F.; Kim, J.; Hess, J.M.; Kübler, K.; Grimsby, J.; Frazer, R.; Zhang, H.; Haradhvala, N.J.; Rosebrock, D.; et al. (2019) RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 2019, 364, eaaw0726. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, T.; Jia, Y.; Luo, X.; Gopal, P.; Li, L.; Odewole, M.; Renteria, V.; Singal, A.G.; Jang, Y.; et al. Somatic mutations increase hepatic clonal fitness and regeneration in chronic liver disease. Cell 2019, 177, 608–621.e12. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? Int. J. Mol. Sci. 2022, 23, 13217. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Compensatory estrogen signal is capable of DNA repair in antiestrogen-responsive cancer cells via activating mutations. J of Oncol 2020; Article ID 5418365.
- Coelingh-Bennink, H.J.; Verhoeven, C.; Dutman, A.E.; Thijssen, J. The use of high-dose estrogens for the treatment of breast cancer. Maturitas 2017, 95, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Rogan, E.; Cavalieri, E. Mechanism of metabolic activation and DNA adduct formation by the human carcinogen diethylstilbestrol: The defining link to natural estrogens. Int J Cancer 2009, 124, 1276–84. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi-Clarke, L.; de Assis, S.; Warri, A. Exposures to Synthetic Estrogens at Different Times During the Life, and Their Effect on Breast Cancer Risk. J Mammary Gland Biol Neoplasia. 2013, 18, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Rasanayagam, S.; Engel, C.; Rizzo, J. State of the evidence 2017: an update on the connection between breast cancer and the environment. Environ Health 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Amplified crosstalk between estrogen binding and GFR signaling mediated pathways of ER activation drives responses in tumors treated with endocrine disruptors. Recent Pat Anticancer Drug Discov 2018, 13, 428–44. [Google Scholar] [CrossRef]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med 2005; 118(Suppl. 12B): 64-73.
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L. , et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–33. [Google Scholar]
- Anderson, G.L.; Limacher, M.; Assaf, A.R. , et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–12. [Google Scholar]
- Suba, Z. Synthetic Estrogens Deregulate Estrogen Receptors Inducing Thromboembolic Complications and Cancer. In: Topics in Anti-Cancer Research. Vol. 8. Eds: Atta-ur-Rahman and Khurshid Zaman. Bentham Science Publishers 2019, Chapter 2, pp. 44-73. [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Aragaki, A.K.; Manson, J.E.; Stefanick, M.L.; et al. Association of Menopausal Hormone Therapy with Breast Cancer Incidence and Mortality during Long Term Follow-up of Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Pan, K. Breast Cancer Prevention: Time for Change. JCO Oncol Pract 2021, 17, 709–716. [Google Scholar] [CrossRef]
- Cortés, M.E.; Alfaro, A.A. The effects of hormonal contraceptives on glycemic regulation. Linacre Q 2014, 81, 209–18. [Google Scholar] [CrossRef]
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 2017, 377, 2228–39. [Google Scholar] [CrossRef]
- Rosenberg, L.; Boggs, D.A.; Wise, L.A.; Adams-Campbell, L.L.; Palmer, J.R. Oral contraceptive use and estrogen/progesterone receptor-negative breast cancer among African American women. Cancer Epidemiol Biomarkers Prev 2010, 19, 2073–9. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Sullivan-Halley, J. , et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res 2010, 70, 575–87. [Google Scholar] [CrossRef]
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. The Lancet Oncology 2015; 16( 9): 1061–70.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008, 371, 303–14. [Google Scholar] [CrossRef]
- Bosetti, C.; Bravi, F.; Negri, E.; La Vecchia, C. Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis. Hum Reprod Update 2009, 15, 489–98. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Qi, X.; Wang, Q. Long term use of oral contraceptives comprising synthetic estrogens induces an excessive breast cancer risk in BRCA mutation carrier women: a meta-analysis. Clinical and Experimental Obstetrics & Gynecology 2022, 49, 1. [Google Scholar]
- Huber, D.; Seitz, S.; Kast, K.; Emons, G.; Ortmann, O. Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review. Arch Gyn Obst 2020, 301, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef]
- Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–92. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef]
- Gorski, J.J.; Kennedy, R.D.; Hosey, A.M.; Harkin, D.P. The complex relationship between BRCA1 and ERalpha in hereditary breast cancer. Clin Cancer Res 2009, 15, 1514–8. [Google Scholar] [CrossRef]
- Wang, L.; Di, L.J. BRCA1 and estrogen/estrogen receptor in breast cancer: where they interact? Int J Biol Sci 2014, 10, 566–75. [Google Scholar]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J. , et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002, 20, 2310–8. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R. , et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001, 98, 10869–74. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Stefansson, I.M.; Chappuis, P.O. , et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003, 95, 1482–5. [Google Scholar] [CrossRef]
- Spillman, M.A.; Bowcock, A.M. BRCA1 and BRCA2 mRNA levels are coordinately elevated in human breast cancer cells in response to estrogen. Oncogene. 1996, 13, 1639–45. [Google Scholar]
- Suba, Z. Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: preventive and therapeutic implications. OncoTargets Ther 2014, 7, 147–64. [Google Scholar] [CrossRef]
- Fan, S.; Wang, J.; Yuan, R. , et al. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 1999, 284, 1354–6. [Google Scholar]
- Xu, J.; Fan, S.; Rosen, E.M. Regulation of the estrogen-inducible gene expression profile by the breast cancer susceptibility gene BRCA1. Endocrinology 2005, 146, 2031–47. [Google Scholar]
- Ma, Y.; Fan, S.; Hu, C.; Meng, Q.; Fuqua, S.A.; Pestell, R.G.; et al. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Molecular Endocrinology 2010, 24, 76–90. [Google Scholar] [CrossRef]
- Fan, S.; Ma, Y.X.; Wang, C. , et al. p300 Modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res 2002, 62, 141–51. [Google Scholar]
- Wang, C.; Fan, S.; Li, Z.; Fu, M.; Rao, M.; Ma, Y.; et al. Cyclin D1 antagonizes BRCA1 repression of estrogen receptor alpha activity. Cancer research 2005, 65, 6557–67. [Google Scholar] [CrossRef]
- Suba, Z. DNA stabilization by the upregulation of estrogen signaling in BRCA gene mutation carriers. Drug Des Devel Ther 2015, 9, 2663–2675. [Google Scholar]
- Fan, S.; Ma, Y.X.; Wang, C.; Yuan, R.Q.; Meng, Q.; Wang, J.A.; Erdos, M.; Goldberg, I.D.; Webb, P.; Kushner, P.J.; Pestell, R.G.; Rosen, E.M. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 2001, 20, 77–87. [Google Scholar] [CrossRef]
- Oktay, K.; Kim, J.Y.; Barad, D.; Babayev, S.N. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol 2010, 28, 240–4. [Google Scholar] [CrossRef]
- Lin, W.T.; Beattie, M.; Chen, L.M.; Oktay, K.; Crawford, S.L.; Gold, E.B.; Cedars, M.; Rosen, M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer 2013, 119, 1652–9. [Google Scholar]
- Chand, A.L.; Simpson, E.R.; Clyne, C.D. Aromatase expression is increased in BRCA1 mutation carriers. BMC Cancer. 2009, 9, 148. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. Toward a unified concept of mammary carcinogenesis Aldaz M. C. Gould M. N. McLachlan J. Slaga T. J. eds. Progress in Clinical and Biological Research: 1-16, Wiley-Liss New York 1997.
- Hosey, A.M.; Gorski, J.J.; Murray, M.M.; Quinn, J.E.; Chung, W.Y.; Stewart, G.E.; et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst 2007, 99, 1683–94. [Google Scholar] [CrossRef]
- Suba, Z. Activating mutations of ESR1, BRCA1 and CYP19 aromatase genes confer tumor response in breast cancers treated with antiestrogens. Recent Pat Anticancer Drug Discov 2017, 12, 136–147. [Google Scholar] [CrossRef]
- Burga, L.N.; Hu, H.; Juvekar, A.; et al. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res 2011, 13, R30. [Google Scholar] [CrossRef]
- Ghosh, S.; Lu, Y.; Katz, A.; Hu, Y.; Li, R. Tumor suppressor BRCA1 inhibits a breast cancer-associated promoter of the aromatase gene (CYP19) in human adipose stromal cells. Am J Physiol Endocrinol Metab 2007, 292, 246–252. [Google Scholar] [CrossRef]
- Ma, Y.; Hu Ch Riegel, A.T.; Fan, S.; Rosen, E.M. Growth factor signaling pathways modulate BRCA1 repression of estrogen receptor-alpha activity. Mol Endocrinol 2007, 21, 1905–23. [Google Scholar] [CrossRef]
- Zheng, L.; Annab, L.A.; Afshari, C.A.; Lee, W.H.; Boyer, T.G. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci USA. 2001, 98, 9587–92. [Google Scholar] [CrossRef]
- Arnold, A.; Papanikolaou, A. Cyclin D1 in Breast Cancer Pathogenesis. J Clin Oncol 2005, 23, 4215–24. [Google Scholar] [CrossRef]
- Sau, A.; Lau, R.; Cabrita, M.A.; Nolan, E.; Crooks, P.A.; et al. Persistent Activation of NF-κB in BRCA1-Deficient Mammary Progenitors Drives Aberrant Proliferation and Accumulation of DNA Damage Cell Stem Cell 2016, 19, 52-65.
- Maggi, A. Liganded and unliganded activation of estrogen receptor and hormone replacement therapies. Biochim Biophys Acta 2011, 1812, 1054–1060. [Google Scholar] [CrossRef]
- Levin, E.R. Bidirectional Signaling between the Estrogen Receptor and the Epidermal Growth Factor Receptor. Mol Endocrinol 2003, 17, 309–317. [Google Scholar] [CrossRef]
- Ohlsson, C.; Mohan, S.; Sjögren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef]
- Martin, M.B.; Franke, T.F.; Stoica, G.E.; Chambon, F.; Katzenellenbogen, B.S.; Stoica, B.A.; et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 2000, 141, 4503–11. [Google Scholar] [CrossRef]
- Chan, T.W.; Pollak, M.; Huynh, H. Inhibition of insulin-like growth factor signaling pathways in mammary gland by pure antiestrogen ICI 182,780. Clin Cancer Res 2001, 7, 2545–54. [Google Scholar]
- Lee, A.V.; Jackson, J.G.; Gooch, J.L.; Hilsenbeck, S.G.; Coronado-Heinsohn, E.; Osborne, C.K.; et al. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol 1999, 13, 787–96. [Google Scholar] [CrossRef]
- Sato, T.; Wang, G.; Hardy, M.P.; Kurita, T.; Cuncha, G.R.; Cooke, P.S. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology 2002, 143, 2673–9. [Google Scholar] [CrossRef]
- Klotz, D.M.; Hewitt, S.C.; Ciana, P.; Raviscioni, M.; Lindzey, J.K.; Foley, J.; et al. s. Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross talk. J Biol Chem 2002, 277, 8531–7. [Google Scholar] [CrossRef]
- DiAugustine, R.P.; Petrusz, P.; Bell, G.I.; Brown, C.F.; Korach, K.S.; McLachlan, J.A.; et al. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 1988, 122, 2355–63. [Google Scholar] [CrossRef]
- Vignon, F.; Bouton, M.M.; Rochefort, H. Antiestrogens inhibit the mitogenic effect of growth factors on breast cancer cells in the total absence of estrogens. Biochem Biophys Res Commun 1987, 146, 1502–8. [Google Scholar] [CrossRef]
- Curtis, S.W.; Washburn, T.; Sewall, C.; DiAugustine, R.; Lindzey, J.; Couse, J.F.; et al. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 1996, 93, 12626–30. [Google Scholar] [CrossRef]
- Das, S.K.; Tsukamura, H.; Paria, B.C.; Andrews, G.K.; Dey, S.K. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology 1994, 134, 971–81. [Google Scholar] [CrossRef]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–4. [Google Scholar] [CrossRef]
- Bunone, G.; Briand, P.A.; Miksicek, R.J.; Picard, D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J 1996, 15, 2174–83. [Google Scholar] [CrossRef]
- Zwijsen, R.M.; Buckle, R.S.; Hijmans, E.M.; Loomans, C.J.; Bernards, R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev 1998, 12, 3488–98. [Google Scholar] [CrossRef]
- McMahon C, Suthiphongchai T, DiRenzo J, Ewen ME. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA 1999, 96, 5382-7.
- Razandi, M.; Pedram, A.; Parks, S.; Levin, E.R. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 2003, 278, 2701–12. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Hughes, C.C.W.; Levin, E.R. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology J Biol Chem 2002, 277, 50768–75. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Chrysogelos, S.A. Identification and characterization of a negative regulatory element within the epidermal growth factor receptor gene first intron in hormone-dependent breast cancer cells. J Cell Biochem 2002, 85, 601–14. [Google Scholar] [CrossRef] [PubMed]
- deFazio, A.; Chiew, Y.E.; Sini, R.L.; Janes, P.W.; Sutherland, R.L. Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer 2000, 87, 487–98. [Google Scholar] [CrossRef]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–33. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Interplay between insulin resistance and estrogen deficiency as co-activators in carcinogenesis. Pathol Oncol Res 2012, 18, 123–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate-2 induction in ovariectomized diabetic rats. Endocrinology 2005, 146, 4786–94. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, M.; Hedo, J.A.; Yamada, K.M.; Kahn, C.R. The structure of insulin receptor and its subunits. Evidence for multiple non reduced forms and a 210,000 possible proreceptor. J Biol Chem. 1982, 257, 10392–9. [Google Scholar]
- Campello, R.S.; Fátima, L.A.; Barreto-Andrade, J.N.; Lucas, T.F.; Mori, R.C.; Porto, C.S.; Machado, U.F. Estradiol-induced regulation of GLUT4 in 3T3-L1 cells: involvement of ESR1 and AKT activation. J Mol Endocrinol 2017, 59, 257–268. [Google Scholar] [CrossRef]
- Richards, R.G.; DiAugustine, R.P.; Petrusz, P.; Clark, G.C.; Sebastian, J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci USA. 1996, 93, 12002–7. [Google Scholar] [CrossRef]
- Mauro, L.; Salerno, M.; Panno, M.L.; Bellizzi, D.; Sisci, D.; Miglietta, A. , et al. Estradiol increases IRS-1 gene expression and insulin signaling in breast cancer cells. Biochem Biophys Res Commun. 2001, 288, 685–9. [Google Scholar] [CrossRef]
- Medina, R.A.; Meneses, A.M.; Vera, J.C.; Guzman, C.; Nualart, F.; Astuya, A. , et al. Estrogen and progesterone up-regulate glucose transporter expression in ZR-75-1 human breast cancer cells. Endocrinology. 2003, 144, 4527–35. [Google Scholar] [CrossRef]
- Garrido, P.; Morán, J.; Alonso, A.; González, S.; González, C. 17β-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology 2013, 154, 1979–89. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Mahboobifard, F.; Pourgholami, M.H.; Jorjani, M.; Dargahi, L.; et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother 2022, 156, 113808. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Low estrogen exposure and/or defective estrogen signaling induces disturbances in glucose uptake and energy expenditure. J Diabet Metab 2013, 4, 272–81. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Rajsheker, S.; Manka, D.; Blomkalns, A.L.; Chatterjee, T.K.; Stoll, L.L.; Weintraub, N. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 2010, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Alonso, J.; Al-Massadi, O.; Castelao, C.; Peinado, J.R.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. J. Proteom. 2011, 74, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Leclercq, G. Recent insights into the effect of natural and environmental estrogens on mammary development and carcinogenesis. The Int J of Develop Biol 2011, 55, 869–78. [Google Scholar] [CrossRef]
- Wang, H.; Leng, Y.; Gong, Y. Bone Marrow Fat and Hematopoiesis. Front. Endocrinol 2018, 9, 694. [Google Scholar] [CrossRef]
- Link, C.D. Is There a Brain Microbiome? Neuroscience Insights. 2021; 16. [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S. , et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem 2019, 294, 18586–99. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–96. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Luu-The, V. , et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998, 63, 322–328. [Google Scholar] [CrossRef]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.Å.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front Endocrinol (Lausanne) 2022, 13, 828780. [Google Scholar] [CrossRef]
- Dieudonné, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol-Cell Physiol 2004, 286, C655–C661. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J Am Acad Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Wang, P.; Mariman, E.; Renes, J.; Keijer, J. The Secretory Function of Adipocytes in the Physiology of White Adipose Tissue. J Cell Physiol 2008, 216, 3–13. [Google Scholar] [CrossRef]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006, 55, 978–987. [Google Scholar] [CrossRef]
- Simpson, E.R.; Misso, M.; Hewitt, K.N. , et al. Estrogen—the good, the bad, and the unexpected. Endocrine Reviews 2005, 26, 322–330. [Google Scholar]
- Combs, T.P.; Pajvani, U.B.; Berg, A.H. , et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004, 145, 367–383. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S. , et al. The hormone resistin links obesity to diabetes. Nature. 2001, 409, 307–12. [Google Scholar] [CrossRef]
- Suba, Z. Crossroad between obesity and cancer: a defective signaling function of heavily lipid laden adipocytes (Online First). In: Crosstalk in Biological Processes. Ed: El-Esawi MA. InTechOpen, London, May 3rd 2019. 3 May. [CrossRef]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular mechanisms underlying metabolic syndrome: the expanding role of the adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol Cells 2014, 37, 365–371. [Google Scholar] [CrossRef]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Research 2002, 4, 65. [Google Scholar] [CrossRef]
- Ye, J.; McGuinness, O.P. 2013. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab 2013, 304, E466–E477. [Google Scholar] [CrossRef]
- Boucher, J.; Tseng, J.-H.; Kahn, C.R. Insulin and insulin-like growth factor receptors act as ligand-specific amplitude modulators of a common pathway regulating gene. J Biol Chem 2010, 285, 17235–17245. [Google Scholar] [CrossRef]
- Smith, C.L. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 1998, 58, 627–32. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, Q.; Lin, G.; Fu, X.; Zhou, K.; Jiang, P. , et al. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1α to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep 2012, 39, 8177–85. [Google Scholar] [CrossRef] [PubMed]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V. , et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc Natl Acad Sci USA 2014, 111, 4892–7. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes, Obesity and Metabolism. 2012, 14, 58–66. [Google Scholar] [CrossRef]
- Suba, Z. Rosetta Stone for Cancer Cure: Comparison of the Anticancer Capacity of Endogenous Estrogens, Synthetic Estrogens and Antiestrogens. Oncol Rev 2023, 17, 10708. [Google Scholar] [CrossRef]
- Thomas, E.T.; Mar, C.D.; Glasziou, P.; Wright, G.; et al. Prevalence of incidental breast cancer and precursor lesions in autopsy studies: a systematic review and meta-analysis. BMC Cancer. 2017, 17, 808. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int J Mol Sci 2023, 24, 6834. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R. , et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology. 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. , et al. Molecular Portraits of Human Breast Tumours. Nature. 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Matta, J.; Morales, L.; Ortiz, C.; Adams, D.; Vargas, W.; Casbas, P. , et al. Estrogen Receptor Expression Is Associated with DNA Repair Capacity in Breast Cancer. PLoS One 2016, 11, e0152422. [Google Scholar] [CrossRef]
- Hayes DF: Tamoxifen:, Dr. Jekyll and Mr. Hyde? J Natl Cancer Inst 2004, 96, 895–897. [Google Scholar]
- Munzone, E.; Colleoni, M. Optimal management of luminal breast cancer: how much endocrine therapy is long enough? Ther Adv Med Oncol. 2018, 10, 1758835918777437. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Weiss, H.; Lee, A.V.; Schiff, R.; De Placido, S.; Osborne, C.K.; et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 2005, 97, 1254–61. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z. , et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, P.; Roger, P.; Rousseau, E.; Thibault, S.; Romieu, G. , et al. HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: results from a French regional cohort. Cancer Med. 2014, 3, 134–42. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Light deficiency confers breast cancer risk by endocrine disorders. Recent Pat Anticancer Discov 2012, 7, 337–44. [Google Scholar] [CrossRef]
- Chew, V.; Toh, H.; Abastado, J. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. Journal of Oncology 2012, 2312956. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Linares, J.; Marín-Jiménez, J.A.; Badia-Ramentol, J.; Calon, A. Determinants and Functions of CAFs Secretome during Cancer progression and Therapy. Front Cell Dev Biol 2021, 8, 621070. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 2022 Nov;21(11):799-820.
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Pejerrey, S.M.; Dustin, D.; Kim, J.A.; Gu, G.; Rechoum, Y. , et al. The impact of ESR1 mutations on the treatment of metastatic breast cancer. Horm Cancer 2018, 9, 215–28. [Google Scholar] [CrossRef]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V. , et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc Natl Acad Sci USA 2014, 111, 4892–7. [Google Scholar] [CrossRef]
- Lei, J.T.; Gou, X.; Seker, S.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat. 2019, 5, 38. [Google Scholar] [CrossRef]
- Stellato, C.; Porreca, I.; Cuomo, D.; Tarallo, R.; Nassa, G.; Ambrosino, C. The "busy life" of unliganded estrogen receptors. Proteomics. 2016, 16, 288–300. [Google Scholar] [CrossRef]
- Fan, P.; Jordan, V.C. New insights into acquired endocrine resistance of breast cancer. Cancer Drug Resist 2019, 2, 198–209. [Google Scholar] [CrossRef]
- Yu, B.; Wu, K.; Wang, X.; Zhang, J.; Wang, L.; Jiang, Y. , et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis 2018, 9, 1082. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–98. [Google Scholar] [CrossRef]
- Giusti, I.; Francesco, M.; Di D'Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G. , et al. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. Ther. 2018, 19, 722. [Google Scholar]
- Dai, G.; Yao, X.; Zhang, Y.; Gu, J.; Geng, Y.; Xue, F. , et al. Colorectal cancer cell–derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bull. Cancer 2018, 105, 336–49. [Google Scholar] [CrossRef]
- Huan, J.; Hornick, N.I.; Shurtleff, M.J.; Skinner, A.M.; Goloviznina, N.A.; Roberts, C.T. , et al. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res. 2013, 73, 918–929. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int J Mol Sci 2018, 19, 611. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 2010, 17, 135–47. [Google Scholar] [CrossRef]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Research 2002, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yamamoto, Y.; Sakamoto, N.; Shimomura, I.; Kogure, A.; Kumazaki, M. , et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene 2019, 38, 5566–5579. [Google Scholar] [CrossRef]
- Meng, M.; Zhong, K.; Jiang, T.; Liu, Z.; Kwan, H.Y.; Su, T. The current understanding on the impact of KRAS on colorectal cancer. Biomedicine & Pharmacotherapy 2021, 140, 111717. [Google Scholar]
- Dörsam, B.; Bösl, T.; Reiners, K.S.; Barnert, S.; Schubert, R.; Shatnyeva, O. , et al. Hodgkin lymphoma-derived extracellular vesicles change the secretome of fibroblasts toward a CAF phenotype. Front. Immunol. 2018, 9, 1358. [Google Scholar] [CrossRef]
- Dannenfelser, R.; Nome, M.; Tahiri, A.; Ursini-Siegel, J.; Vollan, H.K.M.; Haakensen, V.D.; Helland, A.; Naume, B.; Caldas, C.; Borresen-Dale, A.L. , et al. Data-driven analysis of immune infiltrate in a large cohort of breast cancer and its association with disease progression, er activity, and genomic complexity. Oncotarget. 2017, 8, 57121–33. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miki, Y.; Akahira, J.I.; Moriya, T.; Ohuchi, N.; Sasano, H. Review: Aromatase in human breast carcinoma as a key regulator of intratumoral sex steroid concentrations. Endocrine J 2008, 55, 455–63. [Google Scholar] [CrossRef] [PubMed]
- Bollet, M.A.; Savignoni, A.; De Koning, L.; Tran-Perennou, C.; Barbaroux, C.; Degeorges, A. , et al. Tumor aromatase expres sion as a prognostic factor for local control in young breast cancer patients after breast-conserving treatment. Breast Cancer Res 2009, 11, R54. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Ghosh, S.; Procopio, M.G.; Mazzeo, L.; Bordignon, P.; Ostano, P.; et al. (2018). Androgen receptor functions as transcriptional repressor of cancer-associated fibroblast activation. J. Clin. Invest. 128, 5465–5478.
- Vivacqua, A.; Muoio, M.G.; Miglietta, A.M.; Maggiolini, M. (2019). Differential microRNA landscape triggered by estrogens in cancer associated fibroblasts (CAFs) of primary and metastatic breast tumors. Cancers 2019, 11, 412. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, X.; Li, Z.; Xue, Y. Estrogen facilitates gastric cancer cell proliferation and invasion through promoting the secretion of interleukin-6 by cancer-associated fibroblasts. Int. Immunopharmacol. 2020, 78, 105937. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev Drug Discov 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Xu, L.; Dai, Y.; Teng, Y.; Ma, S.; et al. Multicenter, randomized study of genetically modified recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in cancer patients receiving chemotherapy. Support. Care Cancer 2012; 20, 1875–1884.
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: facilitators of cancer and obesity-induced cancer. Annu Rev Cancer Biol. 2021, 5, 17–38. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Haabeth, O.A.; Lorvik, K.B.; Hammarstrom, C.; Donaldson, I.M.; Haraldsen, G.; Bogen, B.; Corthay, A. Inflammation driven by tumour-specific th1 cells protects against b-cell cancer. Nat. Commun. 2011, 2, 240. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. Cd4(+) t cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Fish, E.N. The x-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2015, 6, 635. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M. , et als. Association between cd8+ t-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–43. [Google Scholar] [CrossRef]
- Bentrem, D.J.; Jordan, V.C. Role of antiestrogens and aromatase inhibitors in breast cancer treatment. Curr Opin Obstet Gynecol. 2002, 14, 5–12. [Google Scholar] [CrossRef]
- Jordan, V.C.; Lewis-Wambi, J.S.; Patel, R.R.; Kim, H.; Ariazi, E.A. New hypotheses and opportunities in endocrine therapy: amplification of oestrogen-induced apoptosis. Breast. 2009; 18 Suppl 3: S10-7.
- Osborne, C.K. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998, 339, 1609–18. [Google Scholar] [CrossRef]
- Fan, P.; Agboke, F.A.; Cunliffe, H.E.; Ramos, P.; Jordan, V.C. A molecular model for the mechanism of acquired tamoxifen resistance in breast cancer. Eur J Cancer. 2014, 50, 2866–76. [Google Scholar] [CrossRef]
- Tilghman, S.L.; Sabnis, G.; Brodie, A.M.H. Upregulation of AIB1, aromatase and ERα provides long-term estrogen-deprived human breast cancer cells with a mechanistic growth advantage for survival. Horm Mol Biol Clin Investig. 2011, 3, 357–366. [Google Scholar] [CrossRef]
- Ishii, Y.; Waxman, S.; Germain, D. Tamoxifen Stimulates the Growth of Cyclin D1–Overexpressing Breast Cancer Cells by Promoting the Activation of Signal Transducer and Activator of Transcription 3. Cancer Res 2008, 68, 852–60. [Google Scholar] [CrossRef]
- Zhou, Y.; Yau, C.; Joe WGray, J.W.; Chew, K.; Dairkee, S.H.; Moore, D.H. , et al. Enhanced NFκB and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007, 7, 59. [Google Scholar] [CrossRef]
- Frasor, J.; El-Shennawy, L.; Stender, J.D.; Kastrati, I. NFκB Affects Estrogen Receptor Expression and Activity in Breast Cancer through Multiple Mechanisms. Mol Cell Endocrinol. 2015; 418(0 3): 235–239.
- Hayes, E.L.; Lewis-Wambi, J.S. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015, 17, 40. [Google Scholar] [CrossRef]
- Holst, F.; Stahl, P.R.; Ruiz, C.; Hellwinkel, O.; Jehan, Z.; Wendland, M. , et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet 2007, 39, 655–60. [Google Scholar] [CrossRef]
- Tomita, S.; Zhang, Z.; Nakano, M.; Ibusuki, M.; Kawazoe, T.; Yamamoto, Y.; Iwase, H. Estrogen receptor alpha gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci. 2009, 100, 1012–17. [Google Scholar] [CrossRef]
- Magnani, L.; Frige, G.; Gadaleta, R.M.; Corleone, G.; Fabris, S. , et al. Acquired CYP19A1 amplification is an early specific mechanism of aromatase inhibitor resistance in ER alpha metastatic breast cancer. Nat Genet 2017, 49, 444–50. [Google Scholar] [CrossRef]
- Suba, Z. The pitfall of the transient, inconsistent anticancer capacity of antiestrogens and the mechanism of apparent antiestrogen resistance. Drug Des Devel Ther. 2015, 9, 4341–53. [Google Scholar] [CrossRef]
- Jin, K.; Kong, X.; Shah, T.; Penet, M.-F.; Wildes, F.; Sgroi, D.C. , et al. The HOXB7 protein renders breast cancer cells resistant to tamoxifen through activation of the egfr pathway, Proc. Natl. Acad. Sci. 2012, 109, 2736–41. [Google Scholar] [CrossRef]
- Jin, K.; Park, S.; Teo, W.W.; Korangath, P.; Cho, S.S.; Yoshida, T. , et al. HOXB7 is an ERA cofactor in the activation of HER2 and multiple er target genes leading to endocrine resistance. Cancer Discov 2015, 5, 944–59. [Google Scholar] [CrossRef]
- Osborne, C.K.; Bardou, V.; Hopp, T.A.; Chamness, G.C.; Hilsenbeck, S.G. , et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 2003, 95, 353–61. [Google Scholar] [CrossRef]
- Chen, A.C.; Migliaccio, I.; Rimawi, M.; Lopez-Tarruella, S.; Creighton, C.J.; Massarweh, S.; et al. Upregulation of MUCIN4 in ER-positive/HER2-overexpressing breast cancer xenografts with acquired resistance to endocrine and HER2-targeted therapies. Breast Cancer Res. Treat. 2012; 134 (2) 583–593.
- Fan, P.; Wang, J.; Santen, R.J.; Yue, W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 2007, 67, 1352–60. [Google Scholar] [CrossRef]
- Song, R.X.; Barnes, C.J.; Zhang, Z.; Bao, Y.; Kumar, R. , et al. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA 2004, 101, 2076–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Moerkens, M.; Ramaiahgari, S.; de Bont, H.; Price, L.; Meerman, J.; et al. Elevated insulin-like growth factor-1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res 2011, 13, R52. [Google Scholar] [CrossRef]
- Ellis, M.J.; Perou, C.M. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov 2013, 3, 27–34. [Google Scholar] [CrossRef]
- Pejerrey, S.M.; Dustin, D.; Kim, J.A.; Gu, G.; Rechoum, Y.; et al. The impact of ESR1 mutations on the treatment of metastatic breast cancer. Horm Cancer 2018, 9, 215–28. [Google Scholar] [CrossRef]
- Lei, J.T.; Gou, X.; Seker, S.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat. 2019, 5, 38. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Zhang, C.; Chu, J.; Wu, Y.; Yudong Li, Y.; et al. Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat Commun. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C.; Fan, P.; Abderrahman, B.; Maximov, P.Y.; Hawsawi, Y.M.; Bhattacharya, P.; et al. Sex steroid induced apoptosis as a rational strategy to treat anti-hormone resistant breast and prostate cancer. Discov Med. 2016, 21, 411–27. [Google Scholar] [PubMed]
- Mansouri, S.; Farahmand, L.; Hosseinzade, A. ; Eslami-SZ; Majidzadeh-AK Estrogen can restore Tamoxifen sensitivity in breast cancer cells amidst the complex network of resistance. Biomedicine & Pharmacotherapy 2017, 93, 1320–1325. [Google Scholar]
Figure 1.
Rapid response to Tamoxifen (T) induced ER blockade in cancer cells. Rapid translocation of unbound estrogen receptors (ERs) out of the nucleus helps their interactions with membrane associated growth factor receptors; GFRs (IGF1-R, EGFR). Cytoplasmic ERs activated by growth factor receptors initiate rapid transcriptional processes in the nucleus via transcriptional factors (TFs). Growth factor (GF) activated GFRs may also induce unliganded activation on nuclear unbound ERs driving their transcriptional activity. E: estrogen, P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.
Figure 1.
Rapid response to Tamoxifen (T) induced ER blockade in cancer cells. Rapid translocation of unbound estrogen receptors (ERs) out of the nucleus helps their interactions with membrane associated growth factor receptors; GFRs (IGF1-R, EGFR). Cytoplasmic ERs activated by growth factor receptors initiate rapid transcriptional processes in the nucleus via transcriptional factors (TFs). Growth factor (GF) activated GFRs may also induce unliganded activation on nuclear unbound ERs driving their transcriptional activity. E: estrogen, P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.

Figure 2.
Molecular mechanism of tumor response in Tamoxifen (T) treated cancer cells. Increased estradiol (E2) concentration activates newly expressed abundant estrogen receptors (ERs) increasing the expression of estrogen regulated genes. In the meantime, growth factors (GFs) activate growth factor receptors (GFRs) conferring unliganded activation for free nuclear ERs. The predominance of estradiol (E2) bound ERs over T bound ones leads to DNA repair, apoptotic death and clinical tumor response. P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.
Figure 2.
Molecular mechanism of tumor response in Tamoxifen (T) treated cancer cells. Increased estradiol (E2) concentration activates newly expressed abundant estrogen receptors (ERs) increasing the expression of estrogen regulated genes. In the meantime, growth factors (GFs) activate growth factor receptors (GFRs) conferring unliganded activation for free nuclear ERs. The predominance of estradiol (E2) bound ERs over T bound ones leads to DNA repair, apoptotic death and clinical tumor response. P: phosphorylation, N: nucleus, Dotted arrow: activation, Running arrow: inhibition.

Figure 3.
Molecular mechanism of tumor resistance in Tamoxifen (T) treated cancer cells. The liganded activation of abundant nuclear estrogen receptors (ERs) is completely blocked by T binding. Compensatory abundant expression of membrane associated growth factor receptors (GFRs) struggles for the unliganded activation of T bound ERs. However, the T blockade inhibits the restoration of ER signaling resulting in unrestrained proliferation. GF: growth factor, N: nucleus, Running arrow: inhibition.
Figure 3.
Molecular mechanism of tumor resistance in Tamoxifen (T) treated cancer cells. The liganded activation of abundant nuclear estrogen receptors (ERs) is completely blocked by T binding. Compensatory abundant expression of membrane associated growth factor receptors (GFRs) struggles for the unliganded activation of T bound ERs. However, the T blockade inhibits the restoration of ER signaling resulting in unrestrained proliferation. GF: growth factor, N: nucleus, Running arrow: inhibition.

Table 1.
Receptor pattern in breast cancer subtypes reflecting the grade of DNA damage and the concomitant actions for DNA repair.
Table 1.
Receptor pattern in breast cancer subtypes reflecting the grade of DNA damage and the concomitant actions for DNA repair.
| Subtype of breast cancer | Receptor status | Signs of DNA damage | Signs of DNA repair | Proliferative activity | Response to endocrine therapy |
|---|---|---|---|---|---|
| Luminal A type (50-60%) |
ER overexpression PR positive |
no | ER overexpression | low | good in 50% |
| Luminal B type (10%) |
ER positive PR pos/neg HER2 pos/neg |
PR negative | PR negative PR positive HER2 positive |
increased | moderate/inverse |
| HER2 Enriched (20%) |
ER negative PR negative HER2 rich |
ER negative PR negative |
HER2 rich | high | no |
| Triple negative (10%) |
ER negative PR negative HER2 negative |
ER negative PR negative HER2 negative |
no | high | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Somatic Evolution of Cancer: A New Synthesis
Ulfat Baing
et al.
,
2023
Emerging Role of Oxidative Stress on EGFR and OGG1-BER Cross-Regulation: Implications in Thyroid Physiopathology
Carmelo Moscatello
et al.
,
2020
DNA Repair Gene Expression Adjusted by the PCNA Metagene Predicts Survival in Multiple Cancers
Leif Peterson
et al.
,
2019
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








