Preprint
Communication
Cytomegalovirus DNA Loads in Organs of Congenitally Infected Fetus
Altmetrics
Downloads
110
Views
34
Comments
0
A peer-reviewed article of this preprint also exists.
Abstract
Abstract: Congenital cytomegalovirus (cCMV) infection poses significant risks to fetal development, particularly affecting the nervous system. This study reports a fetal autopsy case, examining cCMV infection and focusing on CMV DNA measurements in various fetal organs before formalin fixation, a novel approach for comprehensive CMV DNA evaluations in fetal organs affected by cCMV. A 20-week-old male fetus was diagnosed with cCMV following the detection of CMV DNA in ascites obtained via abdominocentesis in utero. After termination of pregnancy, multiple organs of the fetus, including the cerebrum, thyroid gland, heart, lungs, liver, spleen, kidneys, and adrenal glands, were extracted and examined for CMV DNA loads using real-time polymerase chain reaction. Histopathological examination involved hematoxylin-eosin and CMV-specific immunostaining. A correlation was found between CMV DNA loads and pathology, with higher CMV-infected cell numbers observed in organs positively identified with both staining methods, exhibiting CMV DNA levels of ≥1.0 × 104 copies/mL, compared to those detected solely by CMV-specific immunostaining, where CMV DNA levels ranged from 1.0 x 103 to 1.0 x 104 copies/mL. These results highlight a quantifiable relationship between organ infection extent and CMV DNA concentration, providing insights into cCMV pathogenesis and potentially informing future diagnostic and therapeutic strategies for cCMV infection.
Keywords:
Submitted:
29 March 2024
Posted:
01 April 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
Submitted:
29 March 2024
Posted:
01 April 2024
You are already at the latest version
Alerts
Abstract
Abstract: Congenital cytomegalovirus (cCMV) infection poses significant risks to fetal development, particularly affecting the nervous system. This study reports a fetal autopsy case, examining cCMV infection and focusing on CMV DNA measurements in various fetal organs before formalin fixation, a novel approach for comprehensive CMV DNA evaluations in fetal organs affected by cCMV. A 20-week-old male fetus was diagnosed with cCMV following the detection of CMV DNA in ascites obtained via abdominocentesis in utero. After termination of pregnancy, multiple organs of the fetus, including the cerebrum, thyroid gland, heart, lungs, liver, spleen, kidneys, and adrenal glands, were extracted and examined for CMV DNA loads using real-time polymerase chain reaction. Histopathological examination involved hematoxylin-eosin and CMV-specific immunostaining. A correlation was found between CMV DNA loads and pathology, with higher CMV-infected cell numbers observed in organs positively identified with both staining methods, exhibiting CMV DNA levels of ≥1.0 × 104 copies/mL, compared to those detected solely by CMV-specific immunostaining, where CMV DNA levels ranged from 1.0 x 103 to 1.0 x 104 copies/mL. These results highlight a quantifiable relationship between organ infection extent and CMV DNA concentration, providing insights into cCMV pathogenesis and potentially informing future diagnostic and therapeutic strategies for cCMV infection.
Keywords:
Subject: Medicine and Pharmacology - Epidemiology and Infectious Diseases
1. Introduction
Cytomegalovirus (CMV) is a pathogen associated with mother-to-child transmission that is known to cause damage to the fetal nervous system. Fetal congenital cytomegalovirus (cCMV) infection occurs due to transplacental CMV transmission. CMV can infect various organs, including the brain, eyes, inner ears, salivary glands, thyroid gland, thymus, heart, lungs, stomach, intestine, liver, spleen, pancreas, kidneys, adrenal glands, epididymis, and testis [1,2,3,4]. The kidneys, lungs, liver, and salivary glands are the most commonly affected organs above [5]. In each organ, CMV-infected cells were identified based on the presence of cytomegalic inclusion bodies (CIBs). There are two types of CIBs: intranuclear amphophilic and cytoplasmic basophilic granular inclusion bodies. The former is a better-known diagnostic tool for CMV infections. Additional pathological tools are sometimes used, as well as conventional hematoxylin-eosin (HE) stains, such as CMV-specific immunostaining and CMV DNA in situ hybridization, are sometimes implemented to detect CIBs in infected cells [2,5].
In the current study, we measured CMV DNA in the organs of fetuses with congenital CMV infection using real-time polymerase chain reaction (PCR). To our knowledge, there are no reports on CMV DNA measurements in multiple fetal organs with cCMV infection. In this fetal autopsy case study of cCMV infection, we performed CMV DNA measurements in fetal organ samples before formalin fixation and pathological examination of fetal organs after formalin fixation.
2. Materials and Methods
This study focused on a male fetus at 20 weeks gestational age (GA) who developed ascites at 16 weeks’ GA and underwent abdominocentesis through the maternal abdominal wall at 18 weeks’ GA. CMV DNA was identified in the ascites sample (5.9 x 105 copies/mL), leading to a diagnosis of cCMV infection. Following the decision to terminate the pregnancy, the mother underwent a vaginal abortion. The fetus weighed 476 g and measured 25 cm in length. Both the head and abdominal circumferences were recorded at 18 cm each. Notably, the abdomen was distended, and ascitic fluid totaling 43 mL was extracted.
Purpura was observed throughout the body (Figure 1). The placental weight was 291 g, and the length of the umbilical cord was 23 cm. His mother provided informed consent for the extraction and testing of his organs. Two and a half hours after delivery, we extracted the cerebrum, thyroid gland, thymus, heart, lungs, stomach, intestine, liver, spleen, pancreas, kidneys, and adrenal gland.
We collected 0.1–0.2 g samples from the organs (excluding the thymus, stomach, intestine, and pancreas) and soaked them in formalin. CMV DNA was extracted from samples using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Real-time PCR was performed to measure CMV DNA load as previously described [6]. After formalin fixation of the extracted organs, pathological examinations were performed using HE and CMV-specific immunostaining. We used a Dako anti-CMV monoclonal antibody (clone CCH2 + DDG9) (Agilent, Santa Clara, CA, USA).
3. Results
CMV DNA was detected in all tested organ samples. CMV DNA loads in each organ sample are presented in Table 1. CMV DNA of ≥1.0 × 103 copies/µgDNA was identified in all organ samples. In the samples from the thyroid gland, lungs, liver, and kidneys, CMV DNA levels were found to be ≥1.0 × 104 copies/µgDNA.
The pathological results for each organ are presented in Table 1. CMV-infected cells showed both HE staining and CMV-specific immunostaining in the thyroid gland, lungs, liver, and kidneys (Figure 2). CMV-infected cells were detected only by CMV-specific immunostaining in the cerebrum, thymus, heart, spleen, pancreas, and adrenal glands (Figure 3 and Figure 4). CMV-infected cells were not found in the stomach or intestine with HE staining or CMV-specific immunostaining.
In the tested samples of the organs where CMV-infected cells were found both with HE stains and with CMV-specific immunostainings, CMV DNA of ≥1.0 × 104 copies/µgDNA was detected. Alternatively, in the other tested samples of the organs where CMV-infected cells were found only with CMV-specific immunostainings, CMV DNA of 1.0 × 103-1.0 × 104 copies/µgDNA was detected.
4. Discussion
In the current fetal autopsy case study, a correlation between pathology and CMV DNA loads in the organs of fetuses with cCMV infection was suggested. A report on CMV DNA measurements in multiple organs of human immunodeficiency virus-infected patients (139 organs in 11 patients) has been published [7]. The authors compared CMV DNA loads in organs where CMV-infected cells were identified through HE staining or CMV-specific immunostaining to those organs without such findings. They reported a strong correlation between the presence of CMV-infected cells and the detection of CMV DNA via quantitative competitive PCR in the evaluated organs. Notably, higher CMV DNA loads were observed in organs where CMV-infected cells were present compared to organs where infected cells were absent. This was attributed to the significantly higher number of CMV-infected cells in the organs where the infected cells were detected.
Although we could not compare CMV DNA loads between the organs where CMV-infected cells were found (cerebrum, thyroid gland, heart, lungs, liver, spleen, kidneys, and adrenal glands) and other organs where infected cells were not found (stomach and intestine), the same idea could be used to interpret our findings.
In the present case study, compared to the fetal organs where CMV-infected cells were found only with CMV-specific immunostaining, the other fetal organs where CMV-infected cells were found, both with HE staining and CMV-specific immunostaining, had 10 times more CMV DNA loads. The higher CMV DNA loads might be due to the higher number of CMV-infected cells in the organs of fetuses with cCMV infection. It was hypothesized that the quantity of CMV-infected cells was greater in fetal organs identified by both HE staining and CMV-specific immunostaining, in comparison to organs where CMV-infected cells were detected solely via CMV-specific immunostaining. The subsequent objective involved assessing whether CMV DNA loads in organs identified exclusively through CMV-specific immunostaining, or in those organs where infected cells were not discernible with either HE stains or CMV-specific immunostaining, are elevated.
A limitation of this case study is the inability to examine the correlation between pathology and CMV DNA load across all extracted fetal organs due to the absence of samples from the thymus, stomach, intestine, and pancreas. Future pathological autopsy cases focusing on cCMV infection should include the collection of samples from these organs to fully assess the correlation between pathology and CMV DNA loads in all extracted fetal organs.
In conclusion, we found a potential correlation between pathology and CMV DNA loads in the organs of fetuses with cCMV infection. Exploring this avenue in the future could deepen our understanding of the pathogenesis of cCMV infections and facilitate the development of innovative treatment strategies.
Author Contributions
Conceptualization, K. T.; methodology, M. I. and R. H.; investigation, M. N. and E. T.; resources, A. K.; writing—original draft preparation, K. T.; writing—review and editing, H. T. and E. K.; supervision, T. I.; funding acquisition, T. I. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding. APC was funded by the Clinical Research Program for Child Health and Development of the Japan Agency for Medical Research and Development (AMED) (grant number: 24gn0110061h0003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the case’s parents.
Data Availability Statement
The datasets used and/or analyzed in this case study are available from the corresponding author upon reasonable request.
Acknowledgments
We appreciate the fetal autopsy.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the case study, collection, analyses, or interpretation of data, writing of the manuscript, or the decision to publish the results.
References
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus Infection during Pregnancy: State of the Science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Sawada, H.; Yodoya, N.; Ohashi, H.; Toriyabe, K.; Hanaki, R.; Sugiura, K.; Toyoda, H.; Matsushita, K.; Koike, Y.; et al. Refractory Ileal Perforations in a Cytomegalovirus-Infected Premature Neonate Resolved after Ganciclovir Therapy. Front. Pediatr. 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ben-David, M.; McKenzie, C.; Sandroussi, C. Cytomegalovirus Adrenalitis Mimicking Adrenal Metastasis in an Immunocompetent Patient. J. Surg. Case Rep. 2022, 2022, rjac122. [Google Scholar] [CrossRef] [PubMed]
- Tsuprun, V.; Keskin, N.; Schleiss, M.R.; Schachern, P.; Cureoglu, S. Cytomegalovirus-Induced Pathology in Human Temporal Bones with Congenital and Acquired Infection. Am. J. Otolaryngol. 2019, 40, 102270. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Kim, K.S.; Park, J.W.; Lee, Y.J.; Lee, M.Y.; Lee, M.C.; Park, C.S.; Juhng, S.W.; Choi, C. Congenital Cytomegalovirus Infection: Three Autopsy Case Reports. J. Korean Med. Sci. 2000, 15, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Toriyabe, K.; Morikawa, F.; Minematsu, T.; Ikejiri, M.; Suga, S.; Ikeda, T. Anti-Cytomegalovirus Immunoglobulin M Titer for Congenital Infection in First-Trimester Pregnancy with Primary Infection: A Multicenter Prospective Cohort Study. J. Perinatol. 2017, 37, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Mattes, F.M.; McLaughlin, J.E.; Emery, V.C.; Clark, D.A.; Griffiths, P.D. Histopathological Detection of Owl’s Eye Inclusions Is Still Specific for Cytomegalovirus in the Era of Human Herpesviruses 6 and 7. J. Clin. Pathol. 2000, 53, 612–614. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s), not of the MDPI and/or editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Figure 1.
Appearance of the case before fetal autopsy. His abdomen was distended by ascites, and purpura was observed all over the body.
Figure 1.
Appearance of the case before fetal autopsy. His abdomen was distended by ascites, and purpura was observed all over the body.

Figure 2.
Pathology of the thyroid gland (2a, 2b), lung (2c, 2d), liver (2e, 2f), and kidney (2g, 2h). In all four fetal organs, cytomegalovirus (CMV)-infected cells with cytomegalic inclusion bodies were found both with hematoxylin-eosin stains (upper row) and with CMV-specific immunostainings (Dako anti-CMV monoclonal antibody) (lower row).
Figure 2.
Pathology of the thyroid gland (2a, 2b), lung (2c, 2d), liver (2e, 2f), and kidney (2g, 2h). In all four fetal organs, cytomegalovirus (CMV)-infected cells with cytomegalic inclusion bodies were found both with hematoxylin-eosin stains (upper row) and with CMV-specific immunostainings (Dako anti-CMV monoclonal antibody) (lower row).
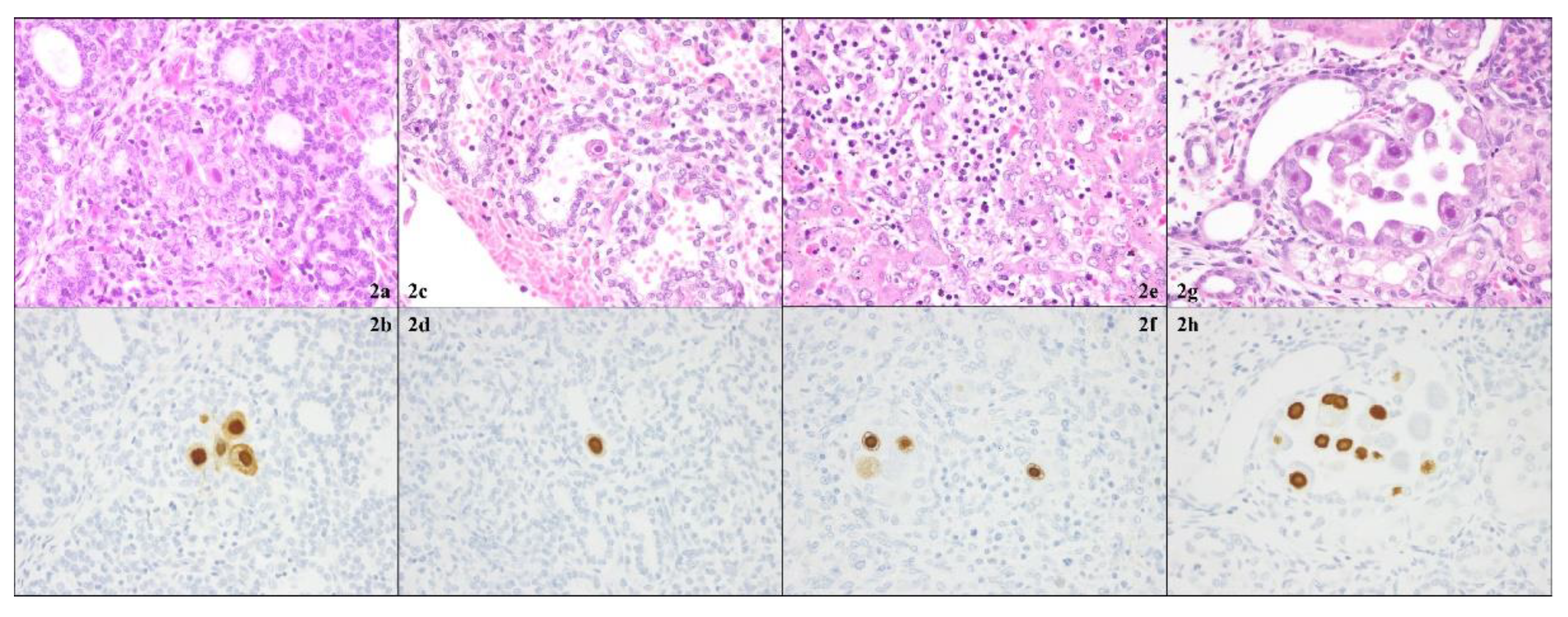
Figure 3.
Pathology of cerebrum (3a, 3b), heart (3c, 3d), spleen (3e, 3f), and adrenal gland (3g, 3h). In all four fetal organs, cytomegalovirus (CMV)-infected cells with cytomegalic inclusion bodies were found only with CMV-specific immunostainings (Dako anti-CMV monoclonal antibody) (lower row).
Figure 3.
Pathology of cerebrum (3a, 3b), heart (3c, 3d), spleen (3e, 3f), and adrenal gland (3g, 3h). In all four fetal organs, cytomegalovirus (CMV)-infected cells with cytomegalic inclusion bodies were found only with CMV-specific immunostainings (Dako anti-CMV monoclonal antibody) (lower row).

Figure 4.
Pathology of thymus (4a, 4b) and pancreas (4c, 4d). In both organs, cytomegalovirus (CMV)-infected cells with cytomegalic inclusion bodies were found only with CMV-specific immunostainings (Dako anti-CMV monoclonal antibody) (lower row).
Figure 4.
Pathology of thymus (4a, 4b) and pancreas (4c, 4d). In both organs, cytomegalovirus (CMV)-infected cells with cytomegalic inclusion bodies were found only with CMV-specific immunostainings (Dako anti-CMV monoclonal antibody) (lower row).

Table 1.
Presence of cytomegalovirus (CMV)-infected cells with hematoxylin-eosin stains and with CMV-specific immunostainings and loads of CMV DNA in each fetal organ sample.
Table 1.
Presence of cytomegalovirus (CMV)-infected cells with hematoxylin-eosin stains and with CMV-specific immunostainings and loads of CMV DNA in each fetal organ sample.
| Fetal organ sample | CMV-infected cells with HE stains | CMV-infected cells with CMV-specific immunostainings | CMV DNA loads (copies/µgDNA) |
|---|---|---|---|
| Thyroid gland | + | + | 5.7 x 104 |
| Lung | + | + | 5.5 x 104 |
| Liver | + | + | 4.9 x 104 |
| Kidney | + | + | 5.7 x 104 |
| Cerebrum | - | + | 1.5 x103 |
| Heart | - | + | 1.1 x103 |
| Spleen | - | + | 1.5 x103 |
| Adrenal gland | - | + | 2.4 x103 |
| Thymus | - | + | NE |
| Pancreas | - | + | NE |
| Stomach | - | - | NE |
| Intestine | - | - | NE |
Cytomegalovirus, CMV, Hematoxylin-eosin, HE, Not examined, NE.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Influence of Gender on CMV Seropositivity in Non-A To G Hepatitis Virus Patients
Rand Farag
et al.
,
2016
How Much Does SARS-CoV-2 Infection during Pregnancy Affect the Neonatal Brain, Heart and Kidney? A Parallel between COVID-19, Vaccination and Normal Pregnancy
Daniela Eugenia Popescu
et al.
,
2024
Post-Vaccination Yellow Fever Antibodies Enhance Zika Virus Infection in Embryoid Bodies
Emily R. Schultz
et al.
,
2020
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








