Preprint
Article
Antagonism and Synergism Characterize the Interactions Between Four North American Potato Virus Y Strains
Altmetrics
Downloads
112
Views
56
Comments
0
A peer-reviewed article of this preprint also exists.
Abstract
Potato virus Y (PVY) is one of the most important constraints to potato production worldwide. There is an increasing occurrence of recombinant PVY strains PVYNTN and PVYN-Wi and a decline in the incidence of the nonrecombinant PVYO. We hypothesized that this may be due to the ability of these recombinant strains to antagonize and/or outcompete PVYO in mixed infections. To deter-mine this, we investigated interactions between PVYO and three recombinant PVY strains com-mon in North America: PVYNTN, PVYN-Wi, and PVYN:O. Overall, our study showed that these inter-actions are tissue dependent. Specifically, PVYNTN, the main causal agent of potato tuber necrotic ringspot disease (PTNRD), was found to be more adaptable than PVYO, especially in potato leaves due, at least in part, to the Ny gene that confers hypersensitive resistance (HR) to PVYO. Further-more, PVYN-Wi was found to repress PVYO in potato tubers but act synergistically in potato leaves. The PVYO-induced foliage necrosis in cultivar ‘Ranger Russet’ was observed to be more severe in plants co-infected by PVYN-Wi and PVYN:O, respectively, resulting in plant death. Strikingly, this PVYO -induced necrosis was suppressed by PVYNTN in doubly infected plants. These interactions may, at least partially, explain the decreasing incidence of PVYO in United States potato produc-tion regions, especially given that many cultivars contain the Ny gene, which likely limits PVYO enabling PVYNTN and PVYN-Wi to outcompete. We also found that replication and cell-to-cell movement of these PVY strains in tubers at 4C was similar to levels at ambient temperature.
Keywords:
Subject: Biology and Life Sciences - Virology
1. Introduction
Potato virus Y (PVY) is one of the most important pathogens of solanaceous plants, such as potato, tobacco, pepper, and ornamental plants, leading to significant losses of the crop [1]. In potato, diseases caused by PVY pose critical challenges to the production of the crop worldwide. PVY is disseminated in potato by aphids and through seed tubers, which are the main mode of potato propagation. The virus exists as a complex of strains that induce a wide variety of foliar and tuber symptoms in potato, leading to yield reductions and loss of tuber quality. PVY evolves through accumulation of mutations and rapidly through recombination between different strains, adapting to new potato cultivars across different environments [2]. Although many PVY recombinants with important genome differences have been identified, almost all these recombinants have PVYO and PVYN as parents [3,4]. In recent decades, new recombinant strains have emerged that have rapidly adapted to the potato ecosystem; these strains continue to dominate virus populations over vast geographical areas in North America [3,5,6]. These recombinants tend to cause milder foliar symptoms; thus, infected plants are less apparent than parent strains, but they cause more severe agricultural losses due to tuber necrosis, which reduces tuber quality. Usual foliar symptoms caused by PVY include leaf mosaic, crinkling, localized necrotic lesions, and leaf drop. Some PVY strains induce distinct ring patterns on the surface of tubers, causing the so-called potato tuber necrotic ringspot disease (PTNRD), which is one of the most damaging viral diseases in potatoes and poses a serious threat to seed and commercial potato production industries [2].
To date, at least nine recombination patterns of PVYO and PVYN sequences have been identified in potato-infecting PVY isolates; the three most common recombinant patterns characteristic of PVY strains are PVYNTN, PVYN:O, and PVYN-Wi [7,8]. PVYN:O and PVYN-Wi are believed to have acquired their recombinant PVYO segments from two separate PVYO lineages, while PVYNTN, which has risen globally and overpopulated other strains worldwide, is thought to have acquired its recombinant PVYO segment from the same lineage as PVYN:O [4].
Recently, the incidence and occurrence of strain PVYNTN has been increasing in the United States seed potato crop while PVYO, the ordinary, non-recombinant strain, has been decreasing [9]. PVYNTN is the main cause of PTNRD in susceptible potato cultivars leading to reductions in tuber quality and quantity [10]. PVYN-Wi has also been reported to cause PTNRD in the United States and Canada [3]. Interestingly, PVYNTN and PVYN-Wi infections are usually either asymptomatic to the foliage or result in transient mild mosaic patterning [2]. On the other hand, PVYO typically causes a strong hypersensitive resistance (HR), including localized necrosis and leaf drop and/or mild mosaic symptoms on the foliage of potato cultivars having the Ny resistance gene. PVYO-induced HR is mediated by the Ny gene, which confers a partial resistance in many North American potato varieties [8]. PVYN:O is less damaging than other strains, but causes mosaic symptoms in susceptible potato varieties [11].
Interactions between PVY strains and potato are defined in large part by resistance genes. Two types of PVY single dominant resistance genes have been identified in potato, namely R genes that confer extreme resistance (ER) and N genes that confer HR [12]. Only a limited number of cultivated potatoes contain R genes, including Rysto from S. stoloniferum [13]. Unlike R genes, N genes occur widely in cultivated potato and the ability of potatoes containing N genes to exhibit the HR upon PVY infection is strain specific [14,15]. Thus, Nctbr and Ncspl from S. tuberosum and S. sparsipilum, respectively, confer HR to PVYC only, and the Nytbr from S. tuberosum confers HR resistance to PVYO isolates only. Strain groups PVYC, PVYO, PVYZ, and PVYD elicit HR phenotypes Nc, Ny, Nz, and Nd, respectively. In addition to PVYO, strains PVYC, PVYD, PVYN, and PVYZ are non-recombinant and serve as parents for many recombinant strains [16,17,18,19]. PVYZ was first described based on its ability to elicit HR in a potato cultivar carrying Nz gene and the inability to induce necrosis in tobacco [20].
There are growing reports that PVYNTN has been spreading rapidly in North America, and that the occurrence of PVYO, the ordinary strain, has been decreasing [2,3,6,9,15]. We therefore hypothesized that antagonistic interactions between PVYO and other PVY strains have led to the decline in importance of the former. In this study, we assessed infectivity of PVYO, PVYNTN, PVYN:O, and PVYN-Wi in N. tabacum and in tubers of potato cultivars ‘Desiree’, ‘Ranger Russet’, ‘Russet Norkotah’ and ‘Eva’, which have different levels of resistance to PVY. Our results indicate a high adaptability of PVYNTN and that it outcompetes PVYO, especially in leaves. Furthermore, PVYN-Wi was shown to antagonize PVYO but act synergistically with PVYNTN and PVYN:O. These findings may, at least partially, explain the current PVY infection dynamics in North America.
2. Materials and Methods
2.1. Virus Inoculations
Virus inoculum was prepared by homogenizing systemically infected leaves of N. tabacum plants in a 0.01 M phosphate buffer, pH 7.0 at a dilution of 1/20 (w/v). Control plants were inoculated with sap from PVY-free N. tabacum cv. Samsun plants. Plants were placed in a growth room at 16 h light and 22 ± 3°C temperature. At five days post infection (dpi), whole inoculated leaves were collected for each of three biological replications, ground to powder in liquid nitrogen and then stored at -80ºC until RNA extraction. Symptoms produced by each strain were recorded for up to 30 dpi.
Four PVY strains from the United States (kindly provided by Stewart Gray of Cornell University) investigated in this study were collected between 2004 and 2006. Three of the strains, PVYO (isolate NY090031; GenBank # KY848009), PVYNTN (isolate NY090029; GenBank #KY848008), and PVYN:O (isolate NY090004; GenBank # KY848007), are isolates from New York State (PVYO, PVYNTN, and PVYN:O), while the fourth strain, PVYN-Wi (isolate MN15_G_52; GenBank # KY847981), was from the state of Minnesota [15]. These isolates were confirmed by RT-PCR of total RNA from tuber samples using strain-specific primers listed in Table 1A. Virus inoculum was then prepared by homogenizing systemically infected leaves (at 14 to 21 dpi) of N. tabacum plants in a 0.01 M phosphate buffer, pH 7.0 at a dilution of 1/20 (w/v). The control was sap from PVY-free N. tabacum plants. PVY strain infectivity was investigated in potato foliage using cultivar ‘Ranger Russet’. The inoculum was prepared as described above. Plants were inoculated at a 4 to 5 leaf stage. Inoculated plants were placed in a room at 16 h light and 22 ± 3°C temperature.
To characterize virus replication and cell-to-cell movement in potato tubers, we investigated three potato cultivars with different levels of resistance to PVY, namely ‘Desiree’ (susceptible), ‘Ranger Russet’ (moderately susceptible) [3] and ‘Eva’ (extreme resistance). All tubers used in these experiments were at the sprouting stage and were tested with reverse transcription polymerase chain reaction (RT-PCR) and confirmed to be PVY-free. To inoculate tubers, a 1 cm corkborer was used to produce a well in the tuber pith (inner medulla) at the center of approximately 2 cm thick sections of potato tubers. Corkborer wells were sealed at one end of the tuber section with an agarose gel in a Petri dish. Virus inoculum was prepared by homogenizing systemically infected leaves of N. tabacum plants in a tuber inoculation buffer (20 mM PBS, 2% PVP, 20 mM Na2SO3, 2 mM EDTA) at a dilution of 1/20 (w/v), followed by centrifugation at 10,000 rpm for 5 minutes. Approximately 150 μl supernatant was placed in the well and the inoculated tubers placed in a closed box and incubated until sample collection, which was conducted using a 0.5 cm corkborer. Samples were taken in triplicate, frozen in liquid nitrogen, and stored at -80ºC until RNA extraction.
2.2. Isolation of RNA and Reverse Transcription Quantitative PCR (RT-qPCR)
Total RNA was isolated from tuber and leaf samples using the RNeasy Mini kit (Qiagen, USA) with modifications. Here, approximately 200 mg of ground leaf and tuber tissues, respectively, were aliquoted for total RNA extraction using a leaf extraction buffer (5 M guanidine thiocyanate, 50 mM Tris-HCl pH 8, 10 mM EDTA, 2% N-lauroylsarcosine) and a tuber RNA extraction buffer (6 M guanidine hydrochloride, 20 mM MES hydrate, 20 mM EDTA, pH 8, 10% β-mercaptoethanol). The quality and quantity of RNA were determined using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, USA) and agarose gel electrophoresis. The Invitrogen SuperScript III Platinum™ SYBR™ green one-step RT-qPCR Kit (Thermo Fisher Scientific, USA) was used to quantify viral RNA in approximately 50 ng of total RNA on a Light Cycler 480 system (Roche Diagnostics). The 10 µl reaction mix contained 5 µl SYBR Green supermix, 0.25 µl SuperScript III reverse transcriptase (RT)/Platinum® Taq DNA polymerase enzyme mix, 0.4 µl 100 µM forward and reverse primers, and 2 µl of total RNA. The RT-qPCR conditions were as follows: cDNA synthesis at 50ºC for 15 min; qPCR cycling at 95ºC for 5 min and 45 three-step cycles, each at 95ºC for 15 sec, 60ºC for 30 sec, and 72ºC for 10 sec, followed by a final melting curve of 65-95ºC for 5 sec. The specificities of primers used in RT-qPCR analyses were determined by running amplification products in an agarose gel and confirming amplification of a single band corresponding to the expected size. Furthermore, amplification efficiencies were determined in a RT-qPCR assay using duplicates of a 10-fold dilution series (four dilutions) as the template.
Mean threshold cycle (Ct) values of the dilution series were plotted against log (1/dilution factor) and the resulting slope value was used to calculate the amplification efficiency (E) using the equation: E=10-1/slope (Rasmussen 2001). Primer pairs with efficiencies ranged from 95 to 109% (Table 1B; Supplementary Figure S1) and therefore within the recommended range [23].
2.3. Determination of Relative Viral RNA Titer
Here, we used elongation factor 1 (EF1) as the housekeeping gene. To compensate for potential variation between runs and plates, EF1 Ct values were normalized by subtracting the median Ct value from individual EF1 Ct values in each replicate. Normalized EF1 Ct values were then subtracted from individual target Ct values to obtain normalized target Ct values, which were used to calculate target ΔCt and ΔΔCt values. In the calculation of relative viral RNA titer, we removed variation from background signals unrelated to viral RNA target by subjecting healthy tissues to the same amplification conditions as for each treatment [24]; this was followed by calculation of mean ΔCt and ΔΔCt of the background noise. These background ΔCt and ΔΔCt values were then subtracted from experimental ΔCt and ΔΔCt values, respectively, the results of which were used to calculate individual 2-ΔΔCt values [25] to obtain relative viral RNA levels. Error bars were computed to indicate the standard deviation of three different technical repeats from three biological replicates, and significant differences were determined using a t-test.
3. Results
3.1. Antagonistic and Synergistic Interactions between PVY Strains in Potato
There are several reports that PVYNTN has been spreading rapidly in North America, and that the occurrence of PVYO, the ordinary strain, has been decreasing [3,6,15,21,26]. We investigated whether this may be due to a PVYNTN competitive advantage over PVYO, during interactions between these viral strains in co-infected plants. We therefore examined co-infections in the potato cultivar ‘Ranger Russet’, which displays an HR upon PVYO infection, due to the presence of the Ny gene [27]. Results showed that leaves of ‘Ranger Russet’ inoculated with PVYO displayed severe local veinal necrosis within 5 dpi in inoculated leaves, and by 10 dpi these symptoms had spread to systemically infected leaves, which displayed yellowing (Figure 1A). By 21 dpi severe veinal and systemic necrosis had spread to all leaves of plants inoculated with PVYO (Supplementary Figure 2A). This PVYO-induced systemic necrosis, which is triggered by the corresponding N gene, resulted in extensive leaf-drop within 30 dpi (Figure 1B). We note that although the Hc-Pro avirulence factor of PVYO restricts virus spread from inoculated leaves of potato cultivars with the Ny gene [27], it has been observed that this resistance is usually overcome, due to other host factors and/or environmental conditions [28,29,30,31,32], resulting in severe systemic necrosis and leaf-drop [15]. As discussed later in this text, these differential symptoms between PVYO, PVYNTN and PVYN-Wi have been shown to be due to Hc-Pro, which has been identified as the PVY avirulence factor that corresponds to Ny tbr and Nc spl [33].
We then investigated whether phenotypes displayed by singly infected plants could be influenced by other PVY strains by doubly inoculating 5 to 6 plants and comparing to symptoms displayed by single infections. Interestingly, in all inoculated plants, the systemic necrosis caused by PVYO was more severe when the latter was co-inoculated with PVYN-Wi, and with PVYN:O, and leaves of these plants displayed severe necrosis and leaf yellowing by 10 dpi (Figure 1A). These symptoms became so severe and resulted in plant death within 30 dpi (Figure 1B; Supplementary Figure S2B). Plant death started from the lower stem and progressed to the apical meristem, which even though wilted had not died at 30 dpi, when observations were discontinued. Also, plants inoculated with PVYN-Wi and PVYN:O displayed systemically infected leaves with a deep orange phenotype (Supplementary Figure S2A). Strikingly, the systemic necrosis and leaf drop caused by PVYO was observed to be absent in plants doubly infected with PVYNTN and PVYO. These plants displayed a phenotype similar to the phenotype displayed by plants singly infected with PVYNTN. Thus, the ability of PVYO to cause systemic necrosis and leaf drop in ‘Ranger Russet’ is repressed by PVYNTN (Figure 1B). Together, these symptoms show that PVYO interacts synergistically with PVYN-Wi and PVYN:O but is antagonized by PVYNTN in ‘Ranger Russet’.
3.2. RT-qPCR Quantification of Viral RNA
To determine the effect of mixed infections on viral RNA levels, viral RNA was quantified in singly and doubly infected plants at 7 dpi from inoculated leaves, and at 30 dpi from newly emerged leaves. RT-qPCR quantification of viral RNA was carried out using strain-specific primers. The specificity of primers used in this study was confirmed by running RT-PCR amplification products on an agarose gel (Supplementary Figure S3). All experiments had 3 biological replicates and three technical replicates. Results showed that at 7 dpi (Figure 2A; a-d), there were almost undetectable levels of PVYO, due likely to the Ny gene in ‘Ranger Russet’ that confers HR, characterized by necrosis to PVYO. Interestingly, in the presence of PVYN-Wi there was a significant increase in the level PVYO RNA. Interactions between PVYN:O and PVYN-Wi, as well as between PVYN:O and PVYO resulted in significantly increased levels of PVYN:O. These increases in viral RNA levels indicate synergism between PVYN:O and PVYN-Wi, as well as PVYN:O and PVYO. The very high RNA levels in plants inoculated with PVYN-Wi and PVYO, and with PVYN:O and PVYN-Wi may at least partly explain plant death in these plants. There were almost undetectable levels of viral RNA in samples collected from the apical meristem of plants inoculated with PVYNTN at 30 dpi (Figure 2A, e-h), consistent with the apparent absence of symptoms in these plants. This is likely due to a deficiency in long distance movement of this strain. We note that although by 30 dpi most of the stem of all plants inoculated with PVYN:O and PVYO, as well as PVYN-Wi and PVYO had died, the apical part of each plant was still in a condition (Figure 1B) for samples to be collected for RNA analysis.
Because ‘Ranger Russet’ has the Ny gene, which confers HR to PVYO, we reasoned that the presence of this gene may have influenced interactions between PVYO and other PVY strains. Thus, we further investigated these interactions in ‘Russet Norkotah’, which does not contain the Ny gene [34]. Samples were collected for viral RNA quantification at 7 and 30 dpi from inoculated and systemic leaves, respectively. Analysis of viral RNA from three biological replicates and three technical replicates at 7 dpi showed that consistent with results recorded in ‘Ranger Russet’, PVYO levels were significantly lower in ‘Russet Norkotah’ leaves when co-inoculated with PVYNTN, PVYN-Wi and PVYN:O, respectively (Figure 2B; i). Similar results were recorded at 30 dpi (Figure 2B; m). Strikingly, unlike result obtained in ‘Ranger Russet’, PVYNTN levels were significantly lower in mixed infections with PVYO (Figure 2B; j, n), indicating that the presence of the Ny gene likely inhibited the ability of PVYO to compete with PVYNTN in ‘Ranger Russet’. Curiously, PVYN-Wi showed very low levels in systemically infected leaves of ‘Russet Norkotah’, except in the presence of PVYNTN at 30 dpi (Figure 2B; o). Together, these results show differential influences on virus replication and movement in the presence of the Ny gene.
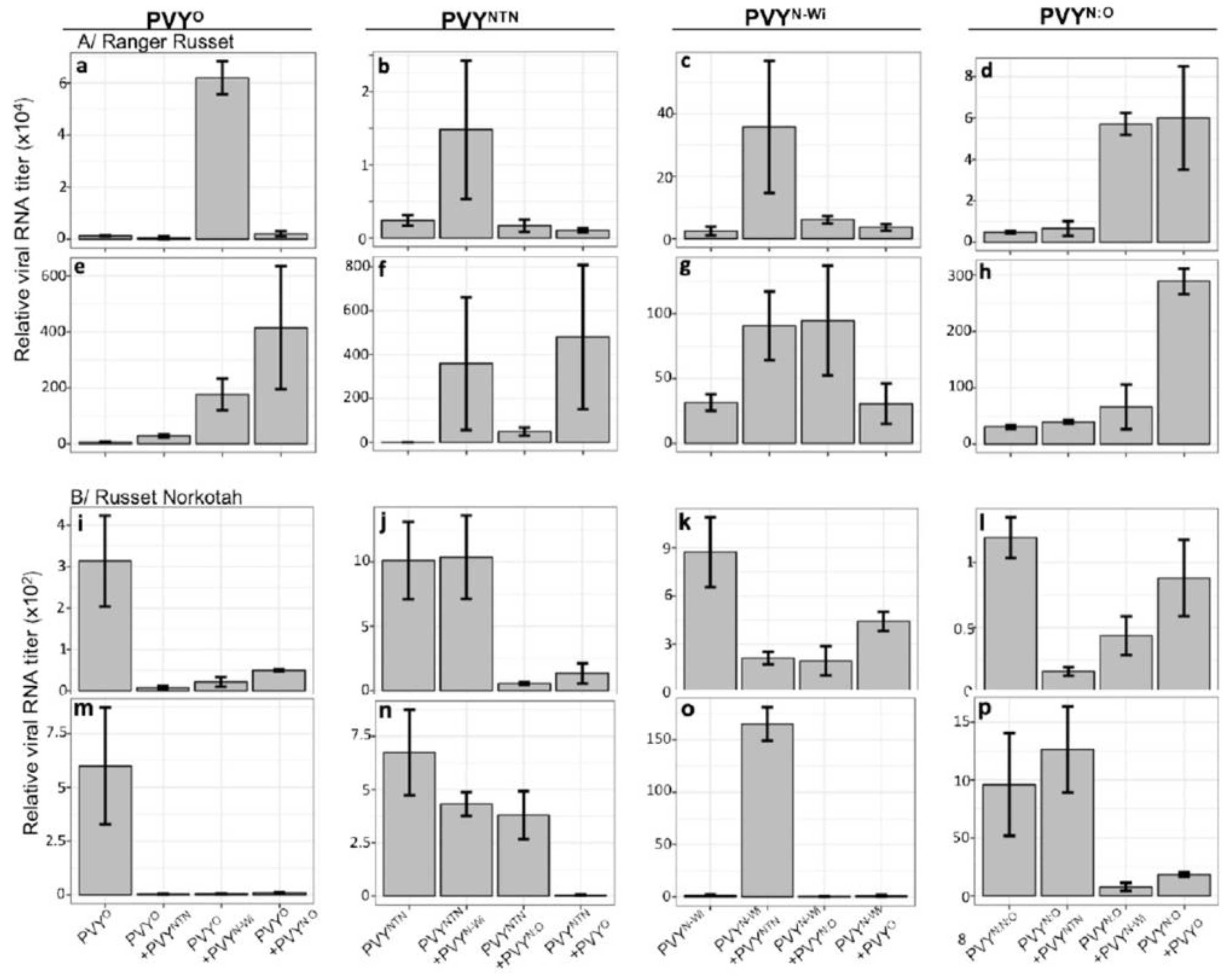
Figure 2.
RT-qPCR quantification of viral RNA in leaves of potato cultivar ‘Ranger Russet’ (A) and ‘Russet Norkotah’ (B) using strain-specific primers. Viral titers were determined at 7 dpi (a-d and i-l) and 30 dpi (e-h and m-p). Each experiment had three biological replicates and three technical replicates. Details of the approach used to determine relative levels of viral RNA are provided in Materials and Methods.
Figure 2.
RT-qPCR quantification of viral RNA in leaves of potato cultivar ‘Ranger Russet’ (A) and ‘Russet Norkotah’ (B) using strain-specific primers. Viral titers were determined at 7 dpi (a-d and i-l) and 30 dpi (e-h and m-p). Each experiment had three biological replicates and three technical replicates. Details of the approach used to determine relative levels of viral RNA are provided in Materials and Methods.
3.3. Replication and Cell-To-Cell Movement of PVY Strains
Potato infecting viruses, as well as viruses of other tuberous root crops tend to spend more time in tubers than elsewhere in the plant. Thus, here, we investigated the ability of all four PVY strains to replicate and move in tubers using tubers of three potato cultivars with different levels of resistance to PVY. Two of the cultivars, ‘Desiree’, and ‘Ranger Russet’, contain N genes [27], while the third cultivar, ‘Eva’, contains the Ryadg extreme resistance gene from S. tuberosum subsp andigena [35]. A key limitation in analyzing potato tuber RNA is the difficulty of extracting good quality total RNA using RNA extraction buffers available in conventional and commercial kits. This is because potato tuber starch is highly phosphorylated and the negatively charged phosphate groups repel adjacent starch chains, which facilitates hydration and gelling [36,37,38,39]. This causes the starch to swell into a viscous gel in the presence of the guanidine thiocyanate found in standard RNA extraction kits, thereby making recovery of a supernatant, and RNA isolation, problematic. We therefore developed a tuber RNA extraction buffer containing guanidine hydrochloride and MES hydrate, which gave very high-quality tuber RNA analyzed in this study. A typical ethidium bromide-stained agarose electrophoresis gel of total tuber RNA is shown in Supplementary Figure S4.
To determine tuber infectivity of these PVY strains, we inoculated tubers with sap from N. tabacum infected by each of the four PVY strains at a dilution of 1/20 (w/v), as described in Materials and Methods. Each treatment had three biological replicates, as well as three technical replicates. Inoculated tubers were stored at 22ºC. At 6 hours post inoculation (hpi), samples were collected at 1 cm from the inoculum well using a 0.5 cm corkborer. Total tuber RNA was extracted, and viral RNA quantified with RT-qPCR using universal PVY primers. Results showed that PVY strain infectivity was influenced by the potato genotype (Figure 3). As expected, tubers of ‘Eva’, which contains the PVY extreme resistance gene, displayed the least amount of viral RNA. The level of PVYNTN RNA was significantly higher than that of other strains, except in ‘Desiree’, in which there was no significant difference between PVYNTN and PVYN-Wi levels (Figure 3). Overall, PVYO displayed the least amount of viral RNA, due at least in part, to the presence in these cultivars of the Ny gene, which confers a HR reaction to PVYO infection. Further, except for ‘Eva’, PVYN-Wi displayed significantly higher levels of viral RNA than those inoculated with PVYN:O. These results confirm that the extreme resistance in ‘Eva’ [35] is not limited to aphid transmission through leaves, but also includes replication and cell-to-cell movement in tubers. The low levels of viral RNA in ‘Eva’ compared with levels in the less resistant ‘Ranger Russet’ and ‘Desiree’ shows that the corkborer method can be used to quantify PVY replication in tubers.
3.4. Interactions between PVY Strains in Potato Tubers
There is evidence that the nature of interaction between PVY strains is dependent on the type of tissues co-infected [40]. To determine whether the apparent antagonism exhibited by PVYNTN over PVYO observed in potato foliage occurs in potato tubers, we investigated the effect of mixed infection between these two PVY strains in tubers of ‘Ranger Russet’. Thus, tubers were singly and doubly inoculated with sap from N. tabacum leaves infected by both strains and samples collected at 6-, 24-, 48-, and 96-hours post infection (hpi). To determine the effect of mixed infection on virus replication and cell-to-cell movement at these time points, samples were collected along the infection gradient (from positions “a” to “c”) as illustrated in Figure 4A. Using strain-specific primers, we carried out an RT-qPCR analysis of PVYNTN and PVYO in mixed and singly infected tubers. Within 6 hpi, viral RNA was detected at position “a” (1 cm) but not at position “b”, indicating that the virus was still within 1 cm radius of the site of inoculation. Interestingly, levels of PVYNTN RNA were lower in mixed infections than in singly inoculated tubers, especially from 48 to 96 hpi (Figure 4B). This result suggests antagonism between the two PVY strains in potato tubers. The extent of PVYNTN antagonism on PVYO declined over distance with a decline in PVYNTN levels (Figure 4C), suggesting that the ability of PVYNTN to suppress PVYO is concentration dependent.
We further investigated interaction of other PVY strains in tubers of ‘Ranger Russet’. Thus, tubers were doubly inoculated with pairs of all four strains and viral RNA levels compared between single and co-infections using strain-specific, as well as universal primers. In ‘Ranger Russet’ tubers, results indicated that in tubers, mixed infection between PVYN:O and PVYO appeared not to significantly affect levels of either strain (Figure 5A). Correspondingly, PVYN-Wi levels appeared not to be affected by PVYO, which itself showed a significant decrease when co-infected with PVYN-Wi (Figure 5B). Also, mixed infection between PVYN:O and PVYNTN caused no significant effect on PVYNTN but resulted in an increase in the level of PVYN:O (Figure 5C). Additionally, PVYN-Wi and PVYNTN antagonized each other (Figure 5D). Furthermore, co-infection between PVYN-Wi and PVYN:O showed a decline in PVYN-Wi but did not significantly affect the levels of PVYN:O (Figure 5E). Moreover, except for tubers doubly infected with PVYN-Wi and PVYNTN, all doubly infected tubers recorded a higher viral RNA load than singly infected tubers, as determined with universal primers (Supplementary Figure S5).
To determine whether interactions between PVY strains was influenced by the Ny gene, we investigated these interactions in tubers of ‘Russet Norkotah’, which does not contain the gene. Results showed that PVYN-Wi and PVYNTN, each acted synergistically with PVYO [40,41]. Strikingly, unlike observations in ‘Ranger Russet’, in ‘Russet Norkotah’, there were significantly lower levels of PVYNTN in leaves co-inoculated with PVYO. (Supplementary Figure S6). Thus, PVYO likely represses PVYNTN in the absence of the Ny gene, to which the host exhibits a HR in the presence of PVYO. Levels of PVYN:O were also repressed by PVYNTN and PVYN-Wi, but not by PVYO in ‘Russet Norkotah’ leaves but showed a significant increase in tubers co-infected with PVYNTN.
3.5. Effect of Temperature on PVY Cell-To-Cell Movement in Tubers
To determine whether low seed storage temperature influences PVY replication and cell-to-cell movement in potato tubers, we quantified the levels of PVYO and PVYNTN RNA over time in tubers at 4ºC, and at 22ºC, respectively. Samples were collected at 6, 24, and 48 hpi using a 0.5 cm corkborer at an approximate distance of 5 mm from the inoculation well, at position “a”, as well as at positions “b” and “c” for a total distance of approximately 1.5 cm from the center of the well, as illustrated in Figure 4A. Results of RT-qPCR quantification indicated that under both temperature conditions, viral RNA was detected at position “a” at 6 hpi, thus indicating that both PVY strains were replicating and carrying out cell-to-cell movement. Interestingly, viral RNA levels declined in samples collected from the same position over time, this may be due to tuber antiviral response.
There were differences between the levels of viral RNA in the two strains. For instance, PVYNTN RNA was significantly higher in ‘Desiree’ tubers at 4ºC compared with 22ºC from positions “a” through “c”. No decline was observed in ‘Ranger Russet’ at position “c” (Figure 6A). In ‘Eva’, there was almost no detectable viral RNA beyond position “a”, consistent with the fact that ‘Eva’ has PVY-extreme resistance, which likely involves virus movement. This result contrasts with the high levels of viral RNA observed along the infection gradient of tubers of the more susceptible ‘Desiree’ and ‘Ranger Russet’. Once again, the almost indetectable levels of viral RNA in ‘Eva’, compared with the less resistant cultivars is further evidence of viral replication and validates use of this approach to quantify virus replication in tubers. Consistent with results obtained in infectivity assessment above, we observed lower levels of PVYO in tubers of all three cultivars at both incubation temperatures compared with levels of PVYNTN, especially at 22ºC where ‘Eva’ and ‘Ranger Russet’ showed very low levels of PVYO RNA (Figure 6B). PVYO RNA levels were more uniform in ‘Desiree’ tubers at 22ºC.
3.6. Evidence of Virus Replication in Potato Tubers
To confirm that the viral RNA detected in this study was from a replicating virus, we carried out an RT-PCR targeting each of PVYNTN and PVYO Hc-Pro cistron negative sense strand RNA [(-)ssRNA], which unlike the (+)ssRNA is not packaged in the virion. Here, samples were collected at 6, 24, 48, and 96 hpi and total tuber RNA extracted as described in Material and Methods for reverse transcription of the (-)ssRNA of the Hc-Pro cistron. Reverse transcription was carried out using the Verso 1-step RT- PCR Reddymix kit (Thermo Scientific, USA) at 50ºC for 20 minutes with a primer targeting the (-)ssRNA of the Hc-Pro cistron. The reverse transcriptase was then inactivated for 10 minutes at 95ºC prior to PCR amplification of the cDNA. Two microliters of the reverse transcription reaction were utilized for PCR amplification using the Dreamtaq PCR mastermix kit (Thermo Scientific, USA) and both Hc-Pro forward and reverse primers (Table 1C). Agarose gel analysis of the PCR products showed the presence of an amplicon corresponding to Hc-Pro cistron (Supplementary Figure S7). Given that the inoculum used in this study was crude sap, where viral RNA is naturally degraded by RNAases, the (-)ssRNA obtained in this analysis could only have come from a replicating virus. This result, together with low levels of viral RNA recorded in cultivar ‘Eva’ as compared to RNA levels in the less resistant ‘Ranger Russet” and ‘Desiree’ observed above, indicates that the viral RNA detected in tubers in this study was from a replicating virus. Thus, the corkborer approach can be used to quantify replicating viral RNA in tubers.
4. Discussion
This study showed that the levels of PVYNTN RNA were consistently higher in potato tubers than those of PVYO. The results also showed that during co-infection, PVYNTN outcompeted PVYO in tubers of ‘Ranger Russet’ (Figure 6B) but not in those of ‘Russet Norkotah’. This is likely due to the presence in ‘Ranger Russet’ of Ny gene, which confers resistance to PVYO, but not to PVYNTN. Given that increasing importance of potato cultivars with the Ny gene in commercial potatoes [27,34,42], these interactions may, at least partly, explain the increasing importance in North America of PVYNTN, and to a lesser extent PVYN-Wi [3,14,15,26]. Further, although synergistic interactions are generally between unrelated viruses [43,44,45] and relationships between related viruses are mostly antagonistic (competitive) [43,44], these data show that synergistic interactions do occur in very closely related PVY strains and that these interactions are genotype and indeed tissue dependent. It is likely that there are host factors present in tubers with which proteins of either of the virus strains are interacting. Future studies will need to determine the mechanism of action, including by analyzing differentially expressed genes. Such a study will determine factors involved in PVYNTN induction of potato tuber necrotic ringspot disease (PTNRD).
It has been shown that Hc-Pro is the PVY avirulence factor corresponding to Nytbr and Ncspl [33]. The specific determinant was subsequently mapped to the Aspartic acid residue at position 419 (Asp419) in PVYO Hc-Pro [46]. In PVYN strains, there is Asp419 → Glu mutation. However, breakdown of Nytbr resistance in PVYNTN and PVYN-Wi was attributed to recombination rather than accumulation of point mutations [33]. The ability of PVYNTN and PVYN-Wi to overcome resistance conferred by the Ny gene may, at least partially, explain the antagonism between these strains and PVYO. Thus, PVYNTN and PVYN-Wi overcome the resistance conferred by Ncspl and outcompete PVYO. It must be stressed that necrosis alone may not be sufficient or essential for the restriction of viral movement. Indeed, other host factors have been reported to be involved in the recognition of the avirulence protein or signaling for HR, as well as light intensity and/or quality, influence the outcome of HR [28,29,30,31,32]. Hence, depending on the potato cultivar and environmental conditions, necrotic responses may vary phenotypically and, in addition to local necrosis, PVY may spread causing systemic necrosis and leaf-drop in the plant as observed on ‘Ranger Russet’ cultivar in this study.
Epidemiologically, these data support the hypothesis that the global increase in occurrence of PVYNTN, and to a limited extent, of PVYN-Wi, is at least partially due to the ability of these strains to outcompete PVYO, the hitherto predominant strain. Thus, in mixed infection situations, the fitness cost is more severe on PVYO than on PVYNTN or PVYN-Wi. Furthermore, it has been indicated that in potato cultivars with the Ny gene, such as ‘Ranger Russet’, severe systemic necrosis observed in this study, is beneficial in PVYO management because it leads to developmentally impaired plants, which are unlikely to be used for seed-tubers [2]. Given that a large proportion of cultivated potatoes have the Ny gene [8,27,42], this results in an increasing importance of PVYNTN and PVYN-Wi at the expense of PVYO. Moreover, the fact that there were no apparent viral symptoms in the foliage of potato plants infected by PVYNTN supports the view that much of the spread is likely through tubers. Equally epidemiologically important is the fact that potato plants doubly infected by PVYN-Wi and PVYO, and by PVYN:O and PVYO exhibited severe veinal and systemic necrosis, leading to plant death under our conditions. Thus, these plants are less likely to be used as seed tubers, thereby limiting these viral strains in mixed infected seed tubers.
Differences in fitness have been suggested to be the driving force in mixed infections between pathogens, and underpin the natural selection process [47,48]. For viruses, fitness is the extent to which the virus adapts to the host and can produce infectious progeny (i.e., replicative fitness) [44,49,50,51]. To be infectious, the virus must uncoat and release its genome, express its genes, and replicate. Nascent viral progeny must move cell-to-cell either as the naked genome or as encapsidated particles while evading host defenses [44,52]. The ability of PVYNTN and PVYN-Wi to outcompete PVYO can at least partially be explained by the ability of the Ny gene to interfere with at least one of these stages in the virus life cycle, thereby conferring resistance. This presumably favors the replication and/or spread of PVYNTN and PVYN-Wi.
Potato seed tubers are traditionally stored at low temperatures, usually between 3ºC and 5ºC [53], and then used in the next crop. Therefore, we investigated the effect of low storage temperature on the replication and cell-to-cell movement of PVYNTN and PVYO at 4ºC and 22ºC in tubers of cultivars ‘Desiree’, ‘Ranger Russet’, and ‘Eva’. Results showed similar levels of viral RNA at both temperature regimes. Thus, unlike the pathogenicity of tuber infecting bacterial and fungal diseases, which tend to decrease at low temperatures , PVY replication and cell-to-cell movement appear not to be affected by low storage temperatures. Epidemiologically, this indicates that even for low virus titer at harvest, PVY can continue to replicate and move cell-to-cell in the tuber during storage. It is important to note that others noted not substantial increase in PVY levels during storage [58]. Furthermore, the fact that the higher levels of PVYNTN over PVYO were more significant at 4ºC than at 22ºC indicates that PVYNTN is much fitter during seed tuber storage and may at least partially explain its gradual replacement of PVYO in North America, where seed tubers are traditionally stored at low temperatures.
One of the most limiting factors in studying virus pathogenicity in tubers is the difficulty of carrying out reproducible transmission in tubers. Thus, here, we developed a corkborer method and used it to infect tubers of all three potato cultivars efficiently and reproducibly.
Taken together, this study indicates that the current increase of PVYNTN and PVYN-Wi incidences in potato fields, especially in North America, may be due, at least partially, to their ability to antagonize and/or outcompete other strains, including especially the prevalent PVYO strain with the increasing incorporation of the Ny resistance gene into the potato cultivars since interaction between PVY strains is variety dependent [41].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Plant Biotic Interactions project number 2020-67018-31180.
Acknowledgements
We thank Joanna Friesner for the critical review of the manuscript.
References
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Molecular Plant Pathology 2011, 12, 938–954. [CrossRef]
- Karasev, A. V.; Gray, S.M. Continuous and Emerging Challenges of Potato Virus Y in Potato. Annual Review of Phytopathology 2013, 51, 571–586.
- Gray, S.; De Boer, S.; Lorenzen, J.; Karasev, A.; Whitworth, J.; Nolte, P.; Singh, R.; Boucher, A.; Xu, H. Potato Virus Y: An Evolving Concern for Potato Crops in the United States and Canada. Plant Dis 2010, 94, 1384–1397. [CrossRef]
- Karasev, A.V.; Hu, X.; Brown, C.J.; Kerlan, C.; Nikolaeva, O.V.; Crosslin, J.M.; Gray, S.M. Genetic Diversity of the Ordinary Strain of Potato Virus Y (PVY) and Origin of Recombinant PVY Strains. Phytopathology 2011, 101, 778–785. [CrossRef]
- Green, K.J.; Brown, C.J.; Gray, S.M.; Karasev, A.V. Phylogenetic Study of Recombinant Strains of Potato Virus Y. Virology 2017, 507, 40–52. [CrossRef]
- MacKenzie, T.D.B.; Nie, X.; Bisht, V.; Singh, M. Proliferation of Recombinant PVY Strains in Two Potato-Producing Regions of Canada, and Symptom Expression in 30 Important Potato Varieties with Different PVY Strains. Plant Dis 2019, 103, 2221–2230. [CrossRef]
- Hu, X.; Karasev, A.; … C.B.-J. of G.; 2009, undefined Sequence Characteristics of Potato Virus Y Recombinants. microbiologyresearch.org 2009, 90, 3033–3041. [CrossRef]
- Rowley, J.S.; Gray, S.M.; Karasev, A. V. Screening Potato Cultivars for New Sources of Resistance to Potato Virus Y. American Journal of Potato Research 2014 92:1 2015, 92, 38–48. [CrossRef]
- Carroll, J.E.; Smith, D.M.; Gray, S.M. Preferential Acquisition and Inoculation of PVYNTN over PVYO in Potato by the Green Peach Aphid Myzus Persicae (Sulzer). J Gen Virol 2016, 97, 797–802. [CrossRef]
- Boonham, N.; Walsh, K.; Preston, S.; North, J.; Smith, P.; Barker, I. The Detection of Tuber Necrotic Isolates of Potato Virus Y, and the Accurate Discrimination of PVY(O), PVY(N) and PVY(C) Strains Using RT-PCR. J Virol Methods 2002, 102, 103–112. [CrossRef]
- Nie, B.; Singh, M.; Murphy, A.; Sullivan, A.; Xie, C.; Nie, X. Response of Potato Cultivars to Five Isolates Belonging to Four Strains of Potato Virus Y. Plant Dis 2012, 96, 1422–1429. [CrossRef]
- Gebhardt, C.; Valkonen, J.P. Organization of Genes Controlling Disease Resistance in the Potato Genome. Annu Rev Phytopathol 2001, 39, 79–102. [CrossRef]
- Mestre, P.; Brigneti, G.; Baulcombe, D.C. An Ry-Mediated Resistance Response in Potato Requires the Intact Active Site of the NIa Proteinase from Potato Virus Y. Plant J. 2000, 23, 653–661.
- Dupuis, B.; Bragard, C.; Schumpp, O. Resistance of Potato Cultivars as a Determinant Factor of Potato Virus Y (PVY) Epidemiology. Potato Res. 2019, 62, 123–138. [CrossRef]
- Funke, C.N.; Nikolaeva, O.V.; Green, K.J.; Tran, L.T.; Chikh-Ali, M.; Quintero-Ferrer, A.; Cating, R.A.; Frost, K.E.; Hamm, P.B.; Olsen, N.; et al. Strain-Specific Resistance to Potato Virus Y (PVY) in Potato and Its Effect on the Relative Abundance of PVY Strains in Commercial Potato Fields. Plant Dis 2017, 101, 20–28. [CrossRef]
- Glais, L.; Tribodet, M.; Kerlan, C. Genomic Variability in Potato Potyvirus Y (PVY): Evidence That PVY(N)W and PVY(NTN) Variants Are Single to Multiple Recombinants between PVY(O) and PVY(N) Isolates. Arch Virol 2002, 147, 363–378. [CrossRef]
- Hu, X.; Karasev, A.V.; Brown, C.J.; Lorenzen, J.H. Sequence Characteristics of Potato Virus Y Recombinants. J Gen Virol 2009, 90, 3033–3041. [CrossRef]
- Lorenzen, J.; Nolte, P.; Martin, D.; Pasche, J.S.; Gudmestad, N.C. NE-11 Represents a New Strain Variant Class of Potato Virus Y. Arch Virol 2008, 153, 517–525. [CrossRef]
- Ogawa, T.; Nakagawa, A.; Hataya, T.; Ohshima, K. The Genetic Structure of Populations of Potato Virus Y in Japan; Based on the Analysis of 20 Full Genomic Sequences. Journal of Phytopathology 2012, 160, 661–673. [CrossRef]
- Jones, R. a. C. Strain Group Specific and Virus Specific Hypersensitive Reactions to Infection with Potyviruses in Potato Cultivars. Annals of Applied Biology 1990, 117, 93–105. [CrossRef]
- Mondal, S.; Lin, Y.-H.; Carroll, J.E.; Wenninger, E.J.; Bosque-Pérez, N.A.; Whitworth, J.L.; Hutchinson, P.; Eigenbrode, S.; Gray, S.M. Potato Virus Y Transmission Efficiency from Potato Infected with Single or Multiple Virus Strains. Phytopathology 2017, 107, 491–498. [CrossRef]
- Hühnlein, A.; Drechsler, N.; Steinbach, P.; Thieme, T.; Schubert, J. Comparison of Three Methods for the Detection of Potato Virus Y in Seed Potato Certification. Journal of Plant Diseases and Protection 2013, 120, 57–69.
- Taylor, S.; Wakem, M.; Dijkman, G.; Alsarraj, M.; Nguyen, M. A Practical Approach to RT-qPCR-Publishing Data That Conform to the MIQE Guidelines. Methods 2010, 50, S1-5. [CrossRef]
- Wilhelm, J.; Pingoud, A.; Hahn, M. Validation of an Algorithm for Automatic Quantification of Nucleic Acid Copy Numbers by Real-Time Polymerase Chain Reaction. Analytical Biochemistry 2003, 317, 218–225. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [CrossRef]
- Karasev, A.V.; Gray, S.M. Continuous and Emerging Challenges of Potato Virus Y in Potato. Annu Rev Phytopathol 2013, 51, 571–586. [CrossRef]
- Jones, R.A.C.; Vincent, S.J. Strain-Specific Hypersensitive and Extreme Resistance Phenotypes Elicited by Potato Virus Y Among 39 Potato Cultivars Released in Three World Regions Over a 117-Year Period. Plant Disease 2018, 102, 185–196. [CrossRef]
- Caplan, J.L.; Mamillapalli, P.; Burch-Smith, T.M.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplastic Protein NRIP1 Mediates Innate Immune Receptor Recognition of a Viral Effector. Cell 2008, 132, 449–462. [CrossRef]
- Tian, Y.-P.; Valkonen, J.P.T. Recombination of Strain O Segments to HCpro-Encoding Sequence of Strain N of Potato Virus Y Modulates Necrosis Induced in Tobacco and in Potatoes Carrying Resistance Genes Ny or Nc. Molecular Plant Pathology 2015, 16, 735–747. [CrossRef]
- Jin, H.; Liu, Y.; Yang, K.-Y.; Kim, C.Y.; Baker, B.; Zhang, S. Function of a Mitogen-Activated Protein Kinase Pathway in N Gene-Mediated Resistance in Tobacco. Plant J 2003, 33, 719–731. [CrossRef]
- Komatsu, K.; Hashimoto, M.; Ozeki, J.; Yamaji, Y.; Maejima, K.; Senshu, H.; Himeno, M.; Okano, Y.; Kagiwada, S.; Namba, S. Viral-Induced Systemic Necrosis in Plants Involves Both Programmed Cell Death and the Inhibition of Viral Multiplication, Which Are Regulated by Independent Pathways. Mol Plant Microbe Interact 2010, 23, 283–293. [CrossRef]
- Valkonen, J.P.; Rokka, V.M.; Watanabe, K.N. Examination of the Leaf-Drop Symptom of Virus-Infected Potato Using Anther Culture-Derived Haploids. Phytopathology 1998, 88, 1073–1077. [CrossRef]
- Moury, B.; Caromel, B.; Johansen, E.; Simon, V.; Chauvin, L.; Jacquot, E.; Kerlan, C.; Lefebvre, V. The Helper Component Proteinase Cistron of Potato Virus Y Induces Hypersensitivity and Resistance in Potato Genotypes Carrying Dominant Resistance Genes on Chromosome IV. Mol. Plant Microbe Interact. 2011, 24, 787–797. [CrossRef]
- Rowley, J.S.; Gray, S.M.; Karasev, A.V. Screening Potato Cultivars for New Sources of Resistance to Potato Virus Y. Am. J. Potato Res. 2015, 92, 38–48. [CrossRef]
- Plaisted, R.L.; Halseth, D.E.; Brodie, B.B.; Slack, S.A.; Sieczka, J.B.; Christ, B.J.; Paddock, K.M.; Peck, M.W. Eva:A Midseason Golden Nematode- and Virus-Resistant Variety for Use as Tablestock or Chipstock. Am. J. Pot Res 2001, 78, 65–68. [CrossRef]
- Nazarian-Firouzabadi, F.; Visser, R.G.F. Potato Starch Synthases: Functions and Relationships. Biochemistry and Biophysics Reports 2017, 10, 7–16. [CrossRef]
- Kumar, G.N.M.; Iyer, S.; Knowles, N.R. Extraction of RNA from Fresh, Frozen, and Lyophilized Tuber and Root Tissues. J Agric Food Chem 2007, 55, 1674–1678. [CrossRef]
- Vasanthan, T.; Bergthaller, W.; Driedger, D.; Yeung, J.; Sporns, P. Starch from Alberta Potatoes: Wet-Isolation and Some Physicochemical Properties. Food Research International 1999, 32, 355–365. [CrossRef]
- Wischmann, null; Hamborg Nielsen T, null; Lindberg Moller B, null In Vitro Biosynthesis of Phosphorylated Starch in Intact Potato Amyloplasts. Plant Physiol 1999, 119, 455–462. [CrossRef]
- Mondal, S.; Ghanim, M.; Roberts, A.; Gray, S.M. Different Potato Virus Y Strains Frequently Co-Localize in Single Epidermal Leaf Cells and in the Aphid Stylet. J Gen Virol 2021, 102. [CrossRef]
- Singh, M.; Singh, R.P. Host Dependent Cross-Protection between PVYN, PVY°, and PVA in Potato Cultivars and Solarium Brachycarpum. Canadian Journal of Plant Pathology 1995, 17, 82–86. [CrossRef]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Ny-1 and Ny-2 Genes Conferring Hypersensitive Response to Potato Virus Y (PVY) in Cultivated Potatoes: Mapping and Marker-Assisted Selection Validation for PVY Resistance in Potato Breeding. Mol Breed 2014, 34, 267–271. [CrossRef]
- Syller, J. Facilitative and Antagonistic Interactions between Plant Viruses in Mixed Infections. Mol Plant Pathol 2012, 13, 204–216. [CrossRef]
- Syller, J.; Grupa, A. Antagonistic Within-Host Interactions between Plant Viruses: Molecular Basis and Impact on Viral and Host Fitness. Mol Plant Pathol 2016, 17, 769–782. [CrossRef]
- Tatineni, S.; Riethoven, J.-J.M.; Graybosch, R.A.; French, R.; Mitra, A. Dynamics of Small RNA Profiles of Virus and Host Origin in Wheat Cultivars Synergistically Infected by Wheat Streak Mosaic Virus and Triticum Mosaic Virus: Virus Infection Caused a Drastic Shift in the Endogenous Small RNA Profile. PLoS ONE 2014, 9, e111577. [CrossRef]
- Glais, L.; Faurez, F.; Tribodet, M.; Boulard, F.; Jacquot, E. The Amino Acid 419 in HC-Pro Is Involved in the Ability of PVY Isolate N605 to Induce Necrotic Symptoms on Potato Tubers. Virus Res. 2015, 208, 110–119. [CrossRef]
- Abrams, M. Environmental Grain, Organism Fitness, and Type Fitness. Entangled Life 2014, 127–151. [CrossRef]
- Metcalf, C.J.E.; Birger, R.B.; Funk, S.; Kouyos, R.D.; Lloyd-Smith, J.O.; Jansen, V. a. A. Five Challenges in Evolution and Infectious Diseases. Epidemics 2015, 10, 40–44. [CrossRef]
- Barbour, J.D.; Grant, R.M. The Role of Viral Fitness in HIV Pathogenesis. Curr HIV/AIDS Rep 2005, 2, 29–34. [CrossRef]
- Domingo, E. Mechanisms of Viral Emergence. Vet Res 2010, 41, 38. [CrossRef]
- Wargo, A.R.; Kurath, G. Viral Fitness: Definitions, Measurement, and Current Insights. Curr Opin Virol 2012, 2, 538–545. [CrossRef]
- Elena, S.F.; Lalić, J. Plant RNA Virus Fitness Predictability: Contribution of Genetic and Environmental Factors. Plant Pathology 2013, 62, 10–18. [CrossRef]
- Milling, A.; Meng, F.; Denny, T.P.; Allen, C. Interactions with Hosts at Cool Temperatures, Not Cold Tolerance, Explain the Unique Epidemiology of Ralstonia Solanacearum Race 3 Biovar 2. Phytopathology 2009, 99, 1127–1134. [CrossRef]
- Gachango, E.; Hanson, L.E.; Rojas, A.; Hao, J.J.; Kirk, W.W. Fusarium Spp. Causing Dry Rot of Seed Potato Tubers in Michigan and Their Sensitivity to Fungicides. Plant Dis 2012, 96, 1767–1774. [CrossRef]
- Fiers, M.; Edel-Hermann, V.; Chatot, C.; Le Hingrat, Y.; Alabouvette, C.; Steinberg, C. Potato Soil-Borne Diseases. A Review. Agron. Sustain. Dev. 2012, 32, 93–132. [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M.K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato Dry Rot Disease: Current Status, Pathogenomics and Management. 3 Biotech 2020, 10, 503. [CrossRef]
- Magdalena, G.; Dariusz, M. Losses during Storage of Potato Varieties in Relation to Weather Conditions during the Vegetation Period and Temperatures during Long-Term Storage. Am. J. Potato Res. 2018, 95, 130–138. [CrossRef]
- Fox, A.; Evans, F.; Browning, I. Direct Tuber Testing for Potato Y Potyvirus by Real-Time RT-PCR and ELISA: Reliable Options for Post-Harvest Testing?*. EPPO Bulletin 2005, 35, 93–97. [CrossRef]
Figure 1.
Interactions between PVY strains PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato cultivar ‘Ranger Russet’. Systemically infected leaves of plants inoculated with PVYO displayed severe local veinal necrosis at 10 dpi while leaves of plants inoculated with PVYNTN, PVYN-Wi, and PVYN:O displayed necrotic spots (A). Leaves doubly infected with PVYN-Wi and PVYO, as well as with PVYN:O and PVYO displayed severe necrosis leading leaf death (B).
Figure 1.
Interactions between PVY strains PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato cultivar ‘Ranger Russet’. Systemically infected leaves of plants inoculated with PVYO displayed severe local veinal necrosis at 10 dpi while leaves of plants inoculated with PVYNTN, PVYN-Wi, and PVYN:O displayed necrotic spots (A). Leaves doubly infected with PVYN-Wi and PVYO, as well as with PVYN:O and PVYO displayed severe necrosis leading leaf death (B).
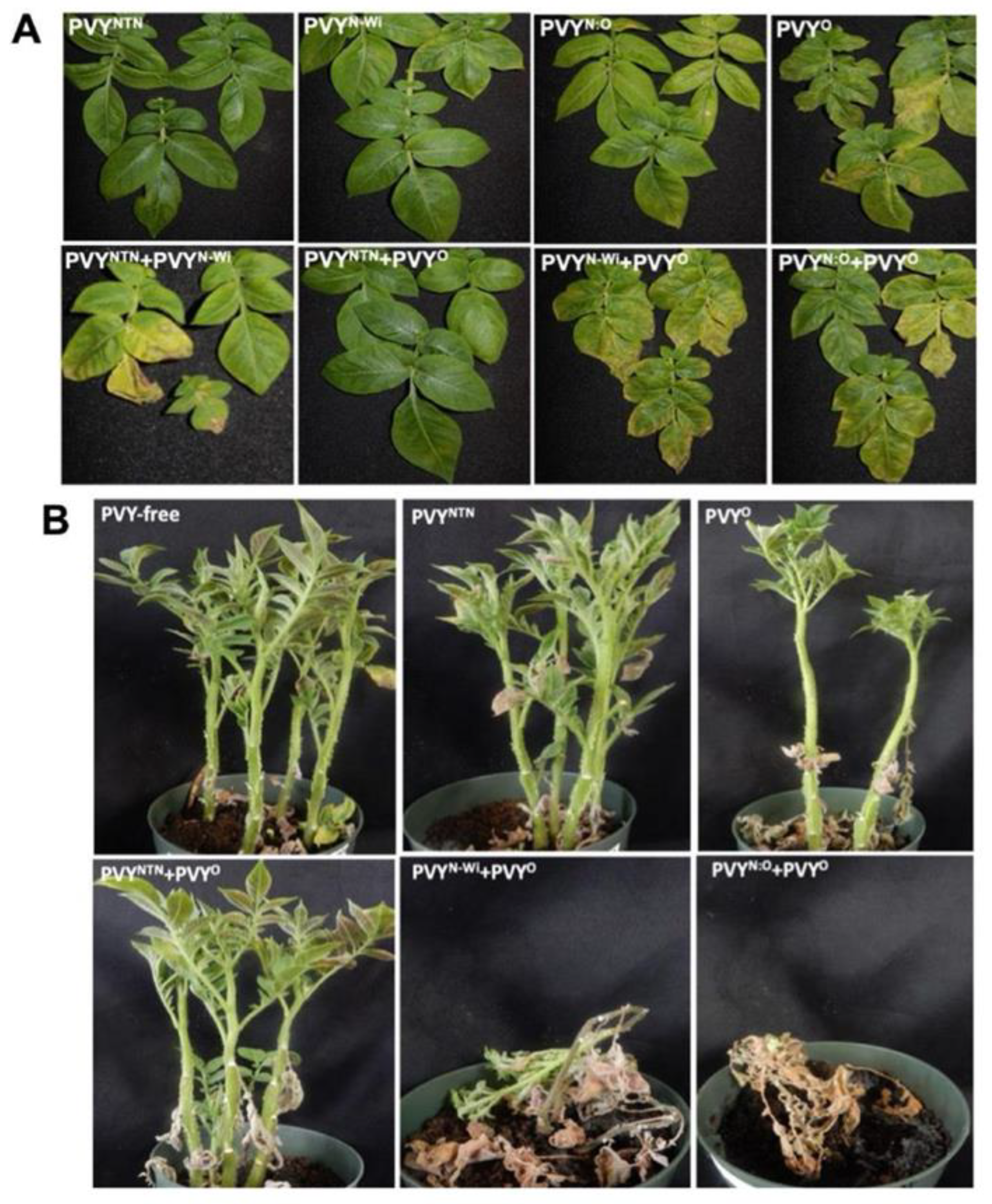
Figure 3.
Replication of four PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in tubers of three potato varieties with different levels of resistance to PVY. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. PVYNTN, and PVYN-Wi to a lesser extent, was observed to replicate to higher levels in ‘Desiree’ and ‘Ranger Russet’ but not in Eva. Mean differences were determined using a t-test.
Figure 3.
Replication of four PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in tubers of three potato varieties with different levels of resistance to PVY. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. PVYNTN, and PVYN-Wi to a lesser extent, was observed to replicate to higher levels in ‘Desiree’ and ‘Ranger Russet’ but not in Eva. Mean differences were determined using a t-test.
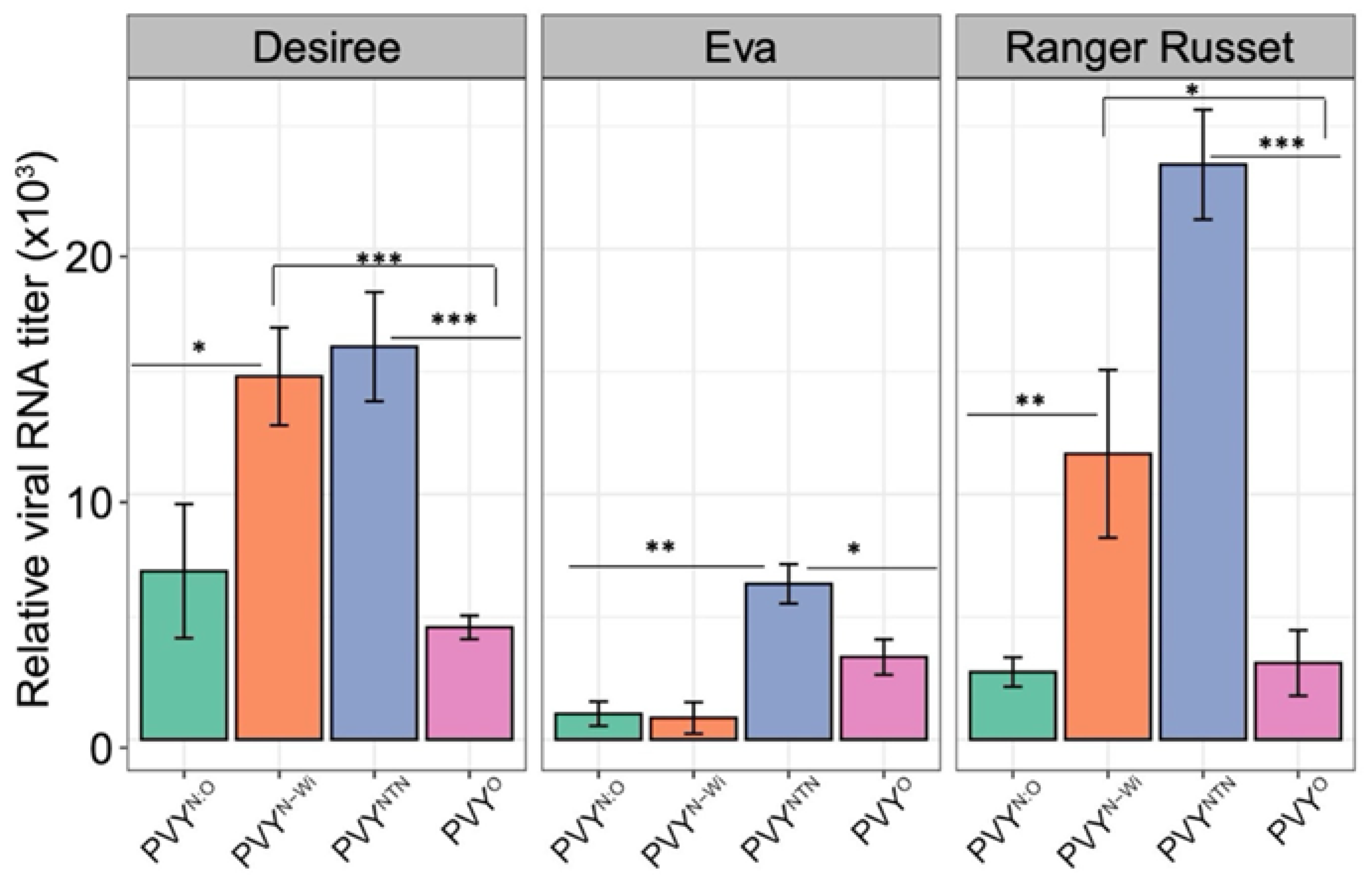
Figure 4.
Quantification of viral RNA in potato tubers at different times (hours) after inoculation. The inoculum was added to the central well using a 1 cm corkborer and samples collected at distances from the inoculum well using a 0.5 cm corkborer and samples collected from positions a, b, and c (4A) at 6, 24, 48, and 96 hours post inoculation. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. Tubers infected by PVYNTN and PVYO, displayed lower levels of viral RNA than tubers singly infected by either of the strains, as indicated by RT-qPCR analysis using strain-specific primers (4B and 4C). Mean differences were determined using a t-test.
Figure 4.
Quantification of viral RNA in potato tubers at different times (hours) after inoculation. The inoculum was added to the central well using a 1 cm corkborer and samples collected at distances from the inoculum well using a 0.5 cm corkborer and samples collected from positions a, b, and c (4A) at 6, 24, 48, and 96 hours post inoculation. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. Tubers infected by PVYNTN and PVYO, displayed lower levels of viral RNA than tubers singly infected by either of the strains, as indicated by RT-qPCR analysis using strain-specific primers (4B and 4C). Mean differences were determined using a t-test.
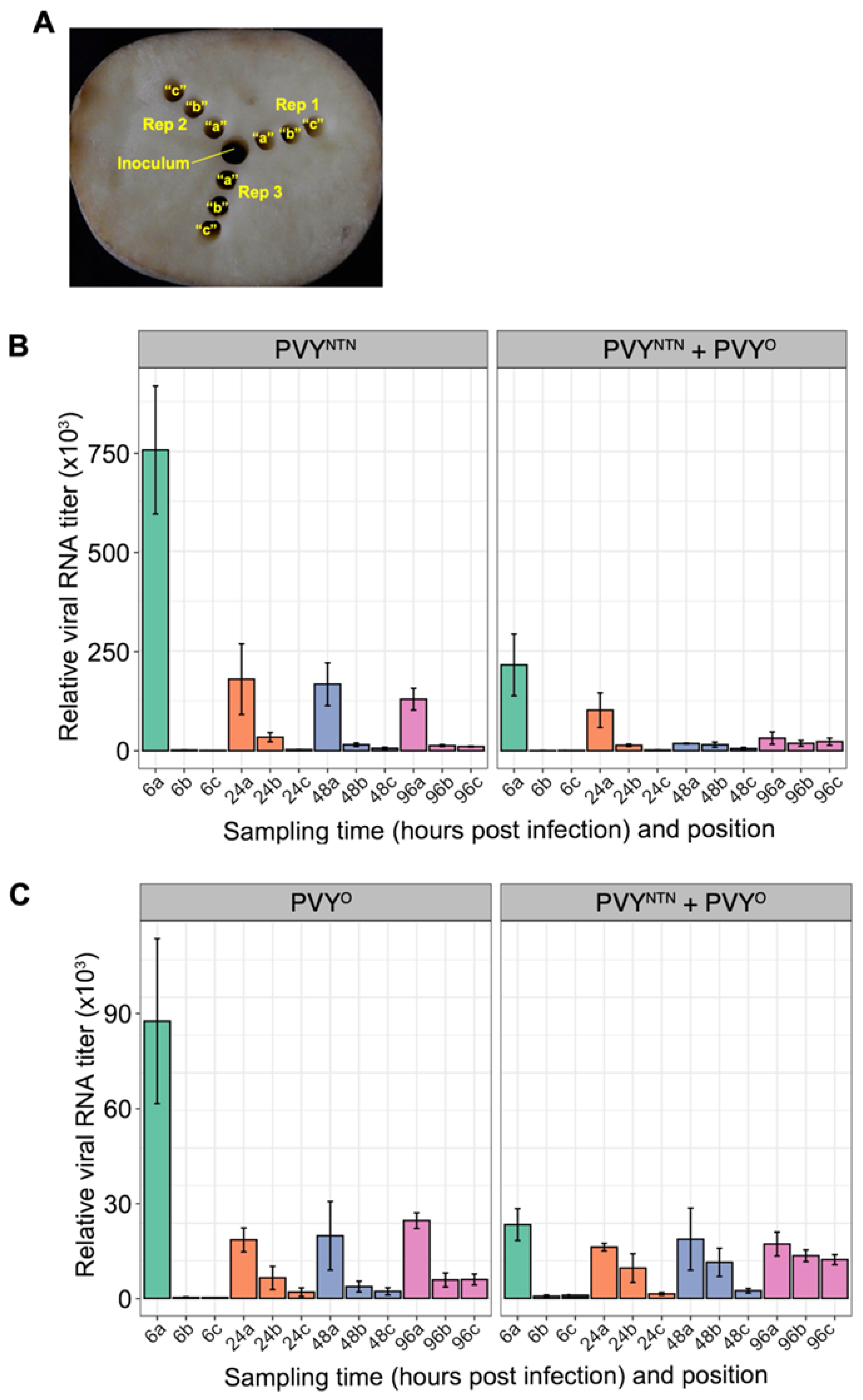
Figure 5.
Antagonism and synergistic interactions between PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato tubers. PVY strains were singly- and co-inoculated to determine the effect of mixed infection on replication, which was determined using RT-qPCR. Relative viral RNA levels were determined as described in Materials and Methods. PVYN-Wi was observed to repress PVYO and PVYNTN but not PVYN:O and the latter in turn represses PVYN-Wi as indicated by strain-specific primers. Mean differences were determined using a t-test.
Figure 5.
Antagonism and synergistic interactions between PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato tubers. PVY strains were singly- and co-inoculated to determine the effect of mixed infection on replication, which was determined using RT-qPCR. Relative viral RNA levels were determined as described in Materials and Methods. PVYN-Wi was observed to repress PVYO and PVYNTN but not PVYN:O and the latter in turn represses PVYN-Wi as indicated by strain-specific primers. Mean differences were determined using a t-test.
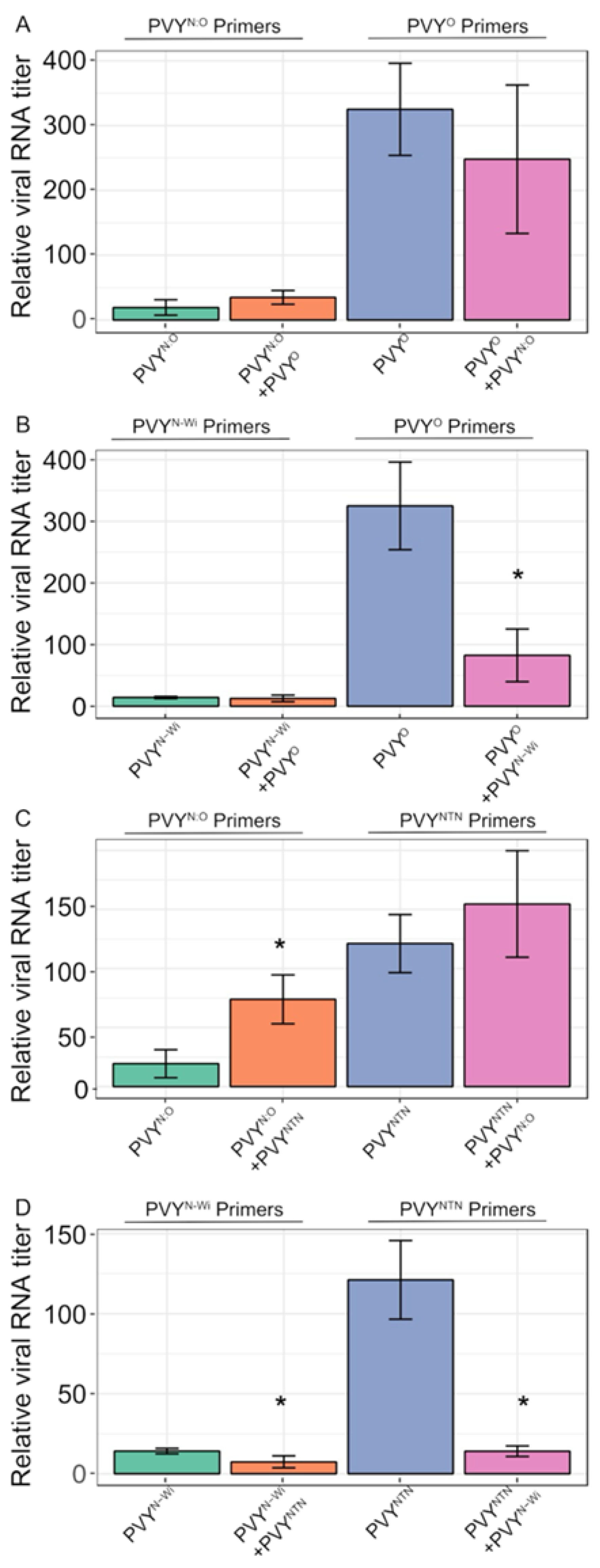
Figure 6.
Effect of temperature on tuber infectivity of PVYNTN (A) and PVYO (B). Tubers of ‘Desiree’, Eva, and ‘Ranger Russet’ were inoculated and incubated at 4ºC and 22ºC, respectively. Samples were collected 6 hpi, 24 hpi, and 48 hpi along the infection gradient from the periphery at ~22 mm (position “a”) from the inoculation well to ~1.5 cm (position “c”). Viral RNA levels were quantified using RT-qPCR as described in Materials and Methods.
Figure 6.
Effect of temperature on tuber infectivity of PVYNTN (A) and PVYO (B). Tubers of ‘Desiree’, Eva, and ‘Ranger Russet’ were inoculated and incubated at 4ºC and 22ºC, respectively. Samples were collected 6 hpi, 24 hpi, and 48 hpi along the infection gradient from the periphery at ~22 mm (position “a”) from the inoculation well to ~1.5 cm (position “c”). Viral RNA levels were quantified using RT-qPCR as described in Materials and Methods.
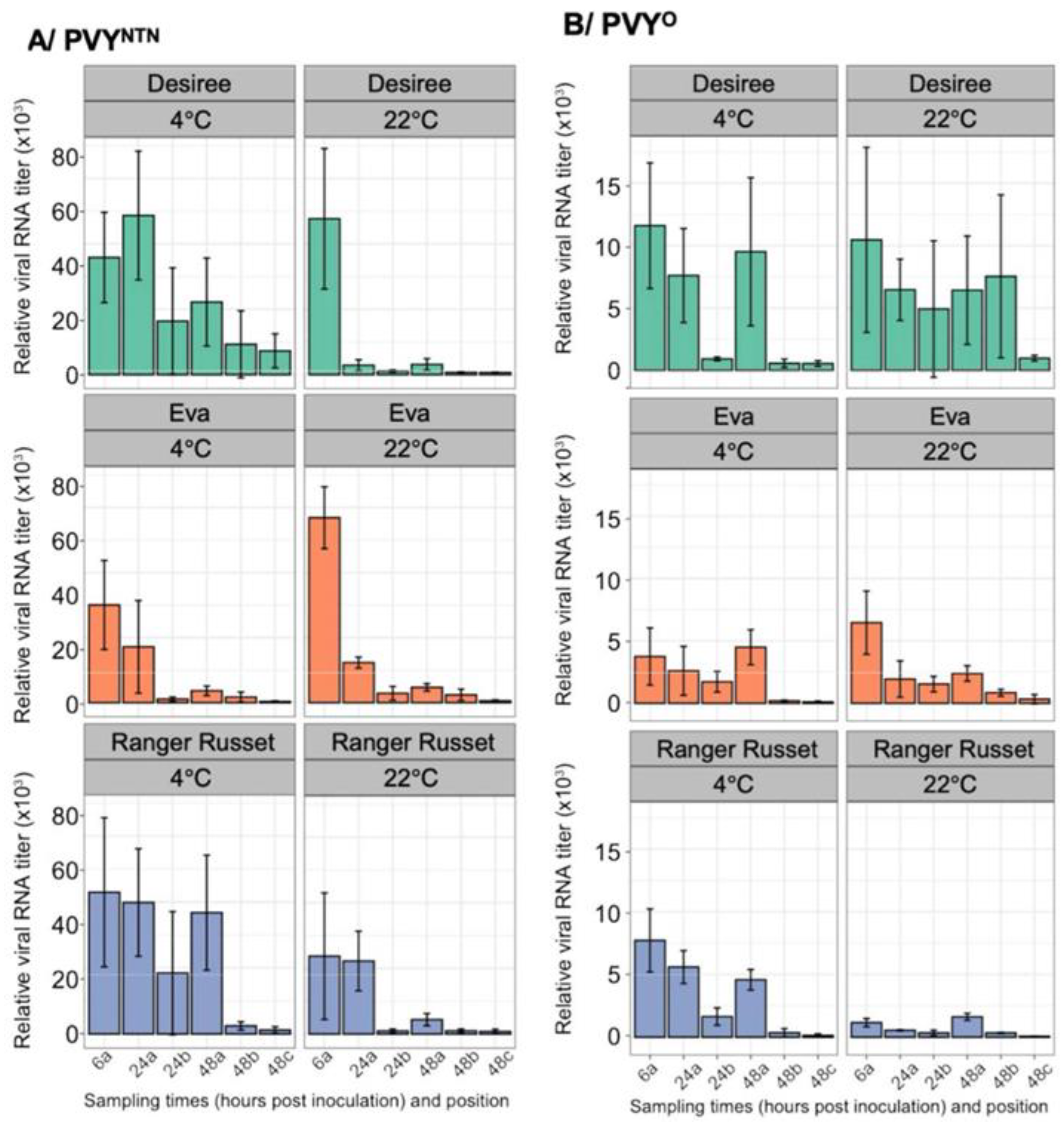
Table 1.
List of primers and targets used in this study.
| A/ RT-PCR | |||
|---|---|---|---|
| Target | Designation | Sequence (5’-3’) | Length (bp) |
| *PVYNTN |
NTN7350-F
NTN8266-R |
ACATCACCGATGAGCAGG
GTACATACCCTCGATTAGCA |
918 |
| *PVYO |
O1962-F
O2296-R |
TCAACATTCTATCCACCAAC
ACGTTTGAGTGTCATGGT |
335 |
| PVYN:O |
N:O1008-F
N:O1703-R |
GCACGTTCCAAGGTTACC
TCGCTTAGCATGATATTCCCT |
695 |
| **PVYN-Wi |
N-Wi_YN5-1780-F
N-Wi_YO3-2558-R |
TCCGAATGGGACAAGAAAACTTG
AGGCTCATCTAACAGCAACTGTC |
778 |
| B/ RT-qPCR*** | |||
| Target | Designation | Sequence (5’-3’) | Length (bp) |
| PVYNTN |
NTNq-6515-F NTNq-6631-R |
TCCGAGCTCCAGTGCAGAAT AAGTGCTGCCCGGTACATTG |
116 |
| PVYO |
Oq-4-F Oq-138-R |
CGCAAAAACACTCATAAAAGCTCA TGGTTGGAAGTGATGAAATTGCT |
134 |
| PVYN:O |
NOq-6444-F NOq-6574-R |
GGATATCATCCTCATCAAATGCCG TCGACGATGCATACTTCTCCTG |
130 |
| PVYN-Wi |
N-Wiq-35-F N-Wiq-156-R |
TTCCTTGCAATTCTCTTAAACGGT ACGAACCGAAACAGATTGTTGAC |
121 |
| All strains |
Uni-q-9426-F
Uni-q-9549-R |
GTGGCAGGGTGATTTCGTCA
AGAATCGCAACATCACCTAATCG |
123 |
| 1-alpha EF1α |
EF1-F EF1-R |
TGGAGGCACTCCCTGGTGACA
TGTGGCAGTCGAGCACTGGT |
194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.pdf (2.74MB )
Submitted:
02 May 2024
Posted:
02 May 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
supplementary.pdf (2.74MB )
Submitted:
02 May 2024
Posted:
02 May 2024
You are already at the latest version
Alerts
Abstract
Potato virus Y (PVY) is one of the most important constraints to potato production worldwide. There is an increasing occurrence of recombinant PVY strains PVYNTN and PVYN-Wi and a decline in the incidence of the nonrecombinant PVYO. We hypothesized that this may be due to the ability of these recombinant strains to antagonize and/or outcompete PVYO in mixed infections. To deter-mine this, we investigated interactions between PVYO and three recombinant PVY strains com-mon in North America: PVYNTN, PVYN-Wi, and PVYN:O. Overall, our study showed that these inter-actions are tissue dependent. Specifically, PVYNTN, the main causal agent of potato tuber necrotic ringspot disease (PTNRD), was found to be more adaptable than PVYO, especially in potato leaves due, at least in part, to the Ny gene that confers hypersensitive resistance (HR) to PVYO. Further-more, PVYN-Wi was found to repress PVYO in potato tubers but act synergistically in potato leaves. The PVYO-induced foliage necrosis in cultivar ‘Ranger Russet’ was observed to be more severe in plants co-infected by PVYN-Wi and PVYN:O, respectively, resulting in plant death. Strikingly, this PVYO -induced necrosis was suppressed by PVYNTN in doubly infected plants. These interactions may, at least partially, explain the decreasing incidence of PVYO in United States potato produc-tion regions, especially given that many cultivars contain the Ny gene, which likely limits PVYO enabling PVYNTN and PVYN-Wi to outcompete. We also found that replication and cell-to-cell movement of these PVY strains in tubers at 4C was similar to levels at ambient temperature.
Keywords:
Subject: Biology and Life Sciences - Virology
1. Introduction
Potato virus Y (PVY) is one of the most important pathogens of solanaceous plants, such as potato, tobacco, pepper, and ornamental plants, leading to significant losses of the crop [1]. In potato, diseases caused by PVY pose critical challenges to the production of the crop worldwide. PVY is disseminated in potato by aphids and through seed tubers, which are the main mode of potato propagation. The virus exists as a complex of strains that induce a wide variety of foliar and tuber symptoms in potato, leading to yield reductions and loss of tuber quality. PVY evolves through accumulation of mutations and rapidly through recombination between different strains, adapting to new potato cultivars across different environments [2]. Although many PVY recombinants with important genome differences have been identified, almost all these recombinants have PVYO and PVYN as parents [3,4]. In recent decades, new recombinant strains have emerged that have rapidly adapted to the potato ecosystem; these strains continue to dominate virus populations over vast geographical areas in North America [3,5,6]. These recombinants tend to cause milder foliar symptoms; thus, infected plants are less apparent than parent strains, but they cause more severe agricultural losses due to tuber necrosis, which reduces tuber quality. Usual foliar symptoms caused by PVY include leaf mosaic, crinkling, localized necrotic lesions, and leaf drop. Some PVY strains induce distinct ring patterns on the surface of tubers, causing the so-called potato tuber necrotic ringspot disease (PTNRD), which is one of the most damaging viral diseases in potatoes and poses a serious threat to seed and commercial potato production industries [2].
To date, at least nine recombination patterns of PVYO and PVYN sequences have been identified in potato-infecting PVY isolates; the three most common recombinant patterns characteristic of PVY strains are PVYNTN, PVYN:O, and PVYN-Wi [7,8]. PVYN:O and PVYN-Wi are believed to have acquired their recombinant PVYO segments from two separate PVYO lineages, while PVYNTN, which has risen globally and overpopulated other strains worldwide, is thought to have acquired its recombinant PVYO segment from the same lineage as PVYN:O [4].
Recently, the incidence and occurrence of strain PVYNTN has been increasing in the United States seed potato crop while PVYO, the ordinary, non-recombinant strain, has been decreasing [9]. PVYNTN is the main cause of PTNRD in susceptible potato cultivars leading to reductions in tuber quality and quantity [10]. PVYN-Wi has also been reported to cause PTNRD in the United States and Canada [3]. Interestingly, PVYNTN and PVYN-Wi infections are usually either asymptomatic to the foliage or result in transient mild mosaic patterning [2]. On the other hand, PVYO typically causes a strong hypersensitive resistance (HR), including localized necrosis and leaf drop and/or mild mosaic symptoms on the foliage of potato cultivars having the Ny resistance gene. PVYO-induced HR is mediated by the Ny gene, which confers a partial resistance in many North American potato varieties [8]. PVYN:O is less damaging than other strains, but causes mosaic symptoms in susceptible potato varieties [11].
Interactions between PVY strains and potato are defined in large part by resistance genes. Two types of PVY single dominant resistance genes have been identified in potato, namely R genes that confer extreme resistance (ER) and N genes that confer HR [12]. Only a limited number of cultivated potatoes contain R genes, including Rysto from S. stoloniferum [13]. Unlike R genes, N genes occur widely in cultivated potato and the ability of potatoes containing N genes to exhibit the HR upon PVY infection is strain specific [14,15]. Thus, Nctbr and Ncspl from S. tuberosum and S. sparsipilum, respectively, confer HR to PVYC only, and the Nytbr from S. tuberosum confers HR resistance to PVYO isolates only. Strain groups PVYC, PVYO, PVYZ, and PVYD elicit HR phenotypes Nc, Ny, Nz, and Nd, respectively. In addition to PVYO, strains PVYC, PVYD, PVYN, and PVYZ are non-recombinant and serve as parents for many recombinant strains [16,17,18,19]. PVYZ was first described based on its ability to elicit HR in a potato cultivar carrying Nz gene and the inability to induce necrosis in tobacco [20].
There are growing reports that PVYNTN has been spreading rapidly in North America, and that the occurrence of PVYO, the ordinary strain, has been decreasing [2,3,6,9,15]. We therefore hypothesized that antagonistic interactions between PVYO and other PVY strains have led to the decline in importance of the former. In this study, we assessed infectivity of PVYO, PVYNTN, PVYN:O, and PVYN-Wi in N. tabacum and in tubers of potato cultivars ‘Desiree’, ‘Ranger Russet’, ‘Russet Norkotah’ and ‘Eva’, which have different levels of resistance to PVY. Our results indicate a high adaptability of PVYNTN and that it outcompetes PVYO, especially in leaves. Furthermore, PVYN-Wi was shown to antagonize PVYO but act synergistically with PVYNTN and PVYN:O. These findings may, at least partially, explain the current PVY infection dynamics in North America.
2. Materials and Methods
2.1. Virus Inoculations
Virus inoculum was prepared by homogenizing systemically infected leaves of N. tabacum plants in a 0.01 M phosphate buffer, pH 7.0 at a dilution of 1/20 (w/v). Control plants were inoculated with sap from PVY-free N. tabacum cv. Samsun plants. Plants were placed in a growth room at 16 h light and 22 ± 3°C temperature. At five days post infection (dpi), whole inoculated leaves were collected for each of three biological replications, ground to powder in liquid nitrogen and then stored at -80ºC until RNA extraction. Symptoms produced by each strain were recorded for up to 30 dpi.
Four PVY strains from the United States (kindly provided by Stewart Gray of Cornell University) investigated in this study were collected between 2004 and 2006. Three of the strains, PVYO (isolate NY090031; GenBank # KY848009), PVYNTN (isolate NY090029; GenBank #KY848008), and PVYN:O (isolate NY090004; GenBank # KY848007), are isolates from New York State (PVYO, PVYNTN, and PVYN:O), while the fourth strain, PVYN-Wi (isolate MN15_G_52; GenBank # KY847981), was from the state of Minnesota [15]. These isolates were confirmed by RT-PCR of total RNA from tuber samples using strain-specific primers listed in Table 1A. Virus inoculum was then prepared by homogenizing systemically infected leaves (at 14 to 21 dpi) of N. tabacum plants in a 0.01 M phosphate buffer, pH 7.0 at a dilution of 1/20 (w/v). The control was sap from PVY-free N. tabacum plants. PVY strain infectivity was investigated in potato foliage using cultivar ‘Ranger Russet’. The inoculum was prepared as described above. Plants were inoculated at a 4 to 5 leaf stage. Inoculated plants were placed in a room at 16 h light and 22 ± 3°C temperature.
To characterize virus replication and cell-to-cell movement in potato tubers, we investigated three potato cultivars with different levels of resistance to PVY, namely ‘Desiree’ (susceptible), ‘Ranger Russet’ (moderately susceptible) [3] and ‘Eva’ (extreme resistance). All tubers used in these experiments were at the sprouting stage and were tested with reverse transcription polymerase chain reaction (RT-PCR) and confirmed to be PVY-free. To inoculate tubers, a 1 cm corkborer was used to produce a well in the tuber pith (inner medulla) at the center of approximately 2 cm thick sections of potato tubers. Corkborer wells were sealed at one end of the tuber section with an agarose gel in a Petri dish. Virus inoculum was prepared by homogenizing systemically infected leaves of N. tabacum plants in a tuber inoculation buffer (20 mM PBS, 2% PVP, 20 mM Na2SO3, 2 mM EDTA) at a dilution of 1/20 (w/v), followed by centrifugation at 10,000 rpm for 5 minutes. Approximately 150 μl supernatant was placed in the well and the inoculated tubers placed in a closed box and incubated until sample collection, which was conducted using a 0.5 cm corkborer. Samples were taken in triplicate, frozen in liquid nitrogen, and stored at -80ºC until RNA extraction.
2.2. Isolation of RNA and Reverse Transcription Quantitative PCR (RT-qPCR)
Total RNA was isolated from tuber and leaf samples using the RNeasy Mini kit (Qiagen, USA) with modifications. Here, approximately 200 mg of ground leaf and tuber tissues, respectively, were aliquoted for total RNA extraction using a leaf extraction buffer (5 M guanidine thiocyanate, 50 mM Tris-HCl pH 8, 10 mM EDTA, 2% N-lauroylsarcosine) and a tuber RNA extraction buffer (6 M guanidine hydrochloride, 20 mM MES hydrate, 20 mM EDTA, pH 8, 10% β-mercaptoethanol). The quality and quantity of RNA were determined using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, USA) and agarose gel electrophoresis. The Invitrogen SuperScript III Platinum™ SYBR™ green one-step RT-qPCR Kit (Thermo Fisher Scientific, USA) was used to quantify viral RNA in approximately 50 ng of total RNA on a Light Cycler 480 system (Roche Diagnostics). The 10 µl reaction mix contained 5 µl SYBR Green supermix, 0.25 µl SuperScript III reverse transcriptase (RT)/Platinum® Taq DNA polymerase enzyme mix, 0.4 µl 100 µM forward and reverse primers, and 2 µl of total RNA. The RT-qPCR conditions were as follows: cDNA synthesis at 50ºC for 15 min; qPCR cycling at 95ºC for 5 min and 45 three-step cycles, each at 95ºC for 15 sec, 60ºC for 30 sec, and 72ºC for 10 sec, followed by a final melting curve of 65-95ºC for 5 sec. The specificities of primers used in RT-qPCR analyses were determined by running amplification products in an agarose gel and confirming amplification of a single band corresponding to the expected size. Furthermore, amplification efficiencies were determined in a RT-qPCR assay using duplicates of a 10-fold dilution series (four dilutions) as the template.
Mean threshold cycle (Ct) values of the dilution series were plotted against log (1/dilution factor) and the resulting slope value was used to calculate the amplification efficiency (E) using the equation: E=10-1/slope (Rasmussen 2001). Primer pairs with efficiencies ranged from 95 to 109% (Table 1B; Supplementary Figure S1) and therefore within the recommended range [23].
2.3. Determination of Relative Viral RNA Titer
Here, we used elongation factor 1 (EF1) as the housekeeping gene. To compensate for potential variation between runs and plates, EF1 Ct values were normalized by subtracting the median Ct value from individual EF1 Ct values in each replicate. Normalized EF1 Ct values were then subtracted from individual target Ct values to obtain normalized target Ct values, which were used to calculate target ΔCt and ΔΔCt values. In the calculation of relative viral RNA titer, we removed variation from background signals unrelated to viral RNA target by subjecting healthy tissues to the same amplification conditions as for each treatment [24]; this was followed by calculation of mean ΔCt and ΔΔCt of the background noise. These background ΔCt and ΔΔCt values were then subtracted from experimental ΔCt and ΔΔCt values, respectively, the results of which were used to calculate individual 2-ΔΔCt values [25] to obtain relative viral RNA levels. Error bars were computed to indicate the standard deviation of three different technical repeats from three biological replicates, and significant differences were determined using a t-test.
3. Results
3.1. Antagonistic and Synergistic Interactions between PVY Strains in Potato
There are several reports that PVYNTN has been spreading rapidly in North America, and that the occurrence of PVYO, the ordinary strain, has been decreasing [3,6,15,21,26]. We investigated whether this may be due to a PVYNTN competitive advantage over PVYO, during interactions between these viral strains in co-infected plants. We therefore examined co-infections in the potato cultivar ‘Ranger Russet’, which displays an HR upon PVYO infection, due to the presence of the Ny gene [27]. Results showed that leaves of ‘Ranger Russet’ inoculated with PVYO displayed severe local veinal necrosis within 5 dpi in inoculated leaves, and by 10 dpi these symptoms had spread to systemically infected leaves, which displayed yellowing (Figure 1A). By 21 dpi severe veinal and systemic necrosis had spread to all leaves of plants inoculated with PVYO (Supplementary Figure 2A). This PVYO-induced systemic necrosis, which is triggered by the corresponding N gene, resulted in extensive leaf-drop within 30 dpi (Figure 1B). We note that although the Hc-Pro avirulence factor of PVYO restricts virus spread from inoculated leaves of potato cultivars with the Ny gene [27], it has been observed that this resistance is usually overcome, due to other host factors and/or environmental conditions [28,29,30,31,32], resulting in severe systemic necrosis and leaf-drop [15]. As discussed later in this text, these differential symptoms between PVYO, PVYNTN and PVYN-Wi have been shown to be due to Hc-Pro, which has been identified as the PVY avirulence factor that corresponds to Ny tbr and Nc spl [33].
We then investigated whether phenotypes displayed by singly infected plants could be influenced by other PVY strains by doubly inoculating 5 to 6 plants and comparing to symptoms displayed by single infections. Interestingly, in all inoculated plants, the systemic necrosis caused by PVYO was more severe when the latter was co-inoculated with PVYN-Wi, and with PVYN:O, and leaves of these plants displayed severe necrosis and leaf yellowing by 10 dpi (Figure 1A). These symptoms became so severe and resulted in plant death within 30 dpi (Figure 1B; Supplementary Figure S2B). Plant death started from the lower stem and progressed to the apical meristem, which even though wilted had not died at 30 dpi, when observations were discontinued. Also, plants inoculated with PVYN-Wi and PVYN:O displayed systemically infected leaves with a deep orange phenotype (Supplementary Figure S2A). Strikingly, the systemic necrosis and leaf drop caused by PVYO was observed to be absent in plants doubly infected with PVYNTN and PVYO. These plants displayed a phenotype similar to the phenotype displayed by plants singly infected with PVYNTN. Thus, the ability of PVYO to cause systemic necrosis and leaf drop in ‘Ranger Russet’ is repressed by PVYNTN (Figure 1B). Together, these symptoms show that PVYO interacts synergistically with PVYN-Wi and PVYN:O but is antagonized by PVYNTN in ‘Ranger Russet’.
3.2. RT-qPCR Quantification of Viral RNA
To determine the effect of mixed infections on viral RNA levels, viral RNA was quantified in singly and doubly infected plants at 7 dpi from inoculated leaves, and at 30 dpi from newly emerged leaves. RT-qPCR quantification of viral RNA was carried out using strain-specific primers. The specificity of primers used in this study was confirmed by running RT-PCR amplification products on an agarose gel (Supplementary Figure S3). All experiments had 3 biological replicates and three technical replicates. Results showed that at 7 dpi (Figure 2A; a-d), there were almost undetectable levels of PVYO, due likely to the Ny gene in ‘Ranger Russet’ that confers HR, characterized by necrosis to PVYO. Interestingly, in the presence of PVYN-Wi there was a significant increase in the level PVYO RNA. Interactions between PVYN:O and PVYN-Wi, as well as between PVYN:O and PVYO resulted in significantly increased levels of PVYN:O. These increases in viral RNA levels indicate synergism between PVYN:O and PVYN-Wi, as well as PVYN:O and PVYO. The very high RNA levels in plants inoculated with PVYN-Wi and PVYO, and with PVYN:O and PVYN-Wi may at least partly explain plant death in these plants. There were almost undetectable levels of viral RNA in samples collected from the apical meristem of plants inoculated with PVYNTN at 30 dpi (Figure 2A, e-h), consistent with the apparent absence of symptoms in these plants. This is likely due to a deficiency in long distance movement of this strain. We note that although by 30 dpi most of the stem of all plants inoculated with PVYN:O and PVYO, as well as PVYN-Wi and PVYO had died, the apical part of each plant was still in a condition (Figure 1B) for samples to be collected for RNA analysis.
Because ‘Ranger Russet’ has the Ny gene, which confers HR to PVYO, we reasoned that the presence of this gene may have influenced interactions between PVYO and other PVY strains. Thus, we further investigated these interactions in ‘Russet Norkotah’, which does not contain the Ny gene [34]. Samples were collected for viral RNA quantification at 7 and 30 dpi from inoculated and systemic leaves, respectively. Analysis of viral RNA from three biological replicates and three technical replicates at 7 dpi showed that consistent with results recorded in ‘Ranger Russet’, PVYO levels were significantly lower in ‘Russet Norkotah’ leaves when co-inoculated with PVYNTN, PVYN-Wi and PVYN:O, respectively (Figure 2B; i). Similar results were recorded at 30 dpi (Figure 2B; m). Strikingly, unlike result obtained in ‘Ranger Russet’, PVYNTN levels were significantly lower in mixed infections with PVYO (Figure 2B; j, n), indicating that the presence of the Ny gene likely inhibited the ability of PVYO to compete with PVYNTN in ‘Ranger Russet’. Curiously, PVYN-Wi showed very low levels in systemically infected leaves of ‘Russet Norkotah’, except in the presence of PVYNTN at 30 dpi (Figure 2B; o). Together, these results show differential influences on virus replication and movement in the presence of the Ny gene.
Figure 2.
RT-qPCR quantification of viral RNA in leaves of potato cultivar ‘Ranger Russet’ (A) and ‘Russet Norkotah’ (B) using strain-specific primers. Viral titers were determined at 7 dpi (a-d and i-l) and 30 dpi (e-h and m-p). Each experiment had three biological replicates and three technical replicates. Details of the approach used to determine relative levels of viral RNA are provided in Materials and Methods.
Figure 2.
RT-qPCR quantification of viral RNA in leaves of potato cultivar ‘Ranger Russet’ (A) and ‘Russet Norkotah’ (B) using strain-specific primers. Viral titers were determined at 7 dpi (a-d and i-l) and 30 dpi (e-h and m-p). Each experiment had three biological replicates and three technical replicates. Details of the approach used to determine relative levels of viral RNA are provided in Materials and Methods.

3.3. Replication and Cell-To-Cell Movement of PVY Strains
Potato infecting viruses, as well as viruses of other tuberous root crops tend to spend more time in tubers than elsewhere in the plant. Thus, here, we investigated the ability of all four PVY strains to replicate and move in tubers using tubers of three potato cultivars with different levels of resistance to PVY. Two of the cultivars, ‘Desiree’, and ‘Ranger Russet’, contain N genes [27], while the third cultivar, ‘Eva’, contains the Ryadg extreme resistance gene from S. tuberosum subsp andigena [35]. A key limitation in analyzing potato tuber RNA is the difficulty of extracting good quality total RNA using RNA extraction buffers available in conventional and commercial kits. This is because potato tuber starch is highly phosphorylated and the negatively charged phosphate groups repel adjacent starch chains, which facilitates hydration and gelling [36,37,38,39]. This causes the starch to swell into a viscous gel in the presence of the guanidine thiocyanate found in standard RNA extraction kits, thereby making recovery of a supernatant, and RNA isolation, problematic. We therefore developed a tuber RNA extraction buffer containing guanidine hydrochloride and MES hydrate, which gave very high-quality tuber RNA analyzed in this study. A typical ethidium bromide-stained agarose electrophoresis gel of total tuber RNA is shown in Supplementary Figure S4.
To determine tuber infectivity of these PVY strains, we inoculated tubers with sap from N. tabacum infected by each of the four PVY strains at a dilution of 1/20 (w/v), as described in Materials and Methods. Each treatment had three biological replicates, as well as three technical replicates. Inoculated tubers were stored at 22ºC. At 6 hours post inoculation (hpi), samples were collected at 1 cm from the inoculum well using a 0.5 cm corkborer. Total tuber RNA was extracted, and viral RNA quantified with RT-qPCR using universal PVY primers. Results showed that PVY strain infectivity was influenced by the potato genotype (Figure 3). As expected, tubers of ‘Eva’, which contains the PVY extreme resistance gene, displayed the least amount of viral RNA. The level of PVYNTN RNA was significantly higher than that of other strains, except in ‘Desiree’, in which there was no significant difference between PVYNTN and PVYN-Wi levels (Figure 3). Overall, PVYO displayed the least amount of viral RNA, due at least in part, to the presence in these cultivars of the Ny gene, which confers a HR reaction to PVYO infection. Further, except for ‘Eva’, PVYN-Wi displayed significantly higher levels of viral RNA than those inoculated with PVYN:O. These results confirm that the extreme resistance in ‘Eva’ [35] is not limited to aphid transmission through leaves, but also includes replication and cell-to-cell movement in tubers. The low levels of viral RNA in ‘Eva’ compared with levels in the less resistant ‘Ranger Russet’ and ‘Desiree’ shows that the corkborer method can be used to quantify PVY replication in tubers.
3.4. Interactions between PVY Strains in Potato Tubers
There is evidence that the nature of interaction between PVY strains is dependent on the type of tissues co-infected [40]. To determine whether the apparent antagonism exhibited by PVYNTN over PVYO observed in potato foliage occurs in potato tubers, we investigated the effect of mixed infection between these two PVY strains in tubers of ‘Ranger Russet’. Thus, tubers were singly and doubly inoculated with sap from N. tabacum leaves infected by both strains and samples collected at 6-, 24-, 48-, and 96-hours post infection (hpi). To determine the effect of mixed infection on virus replication and cell-to-cell movement at these time points, samples were collected along the infection gradient (from positions “a” to “c”) as illustrated in Figure 4A. Using strain-specific primers, we carried out an RT-qPCR analysis of PVYNTN and PVYO in mixed and singly infected tubers. Within 6 hpi, viral RNA was detected at position “a” (1 cm) but not at position “b”, indicating that the virus was still within 1 cm radius of the site of inoculation. Interestingly, levels of PVYNTN RNA were lower in mixed infections than in singly inoculated tubers, especially from 48 to 96 hpi (Figure 4B). This result suggests antagonism between the two PVY strains in potato tubers. The extent of PVYNTN antagonism on PVYO declined over distance with a decline in PVYNTN levels (Figure 4C), suggesting that the ability of PVYNTN to suppress PVYO is concentration dependent.
We further investigated interaction of other PVY strains in tubers of ‘Ranger Russet’. Thus, tubers were doubly inoculated with pairs of all four strains and viral RNA levels compared between single and co-infections using strain-specific, as well as universal primers. In ‘Ranger Russet’ tubers, results indicated that in tubers, mixed infection between PVYN:O and PVYO appeared not to significantly affect levels of either strain (Figure 5A). Correspondingly, PVYN-Wi levels appeared not to be affected by PVYO, which itself showed a significant decrease when co-infected with PVYN-Wi (Figure 5B). Also, mixed infection between PVYN:O and PVYNTN caused no significant effect on PVYNTN but resulted in an increase in the level of PVYN:O (Figure 5C). Additionally, PVYN-Wi and PVYNTN antagonized each other (Figure 5D). Furthermore, co-infection between PVYN-Wi and PVYN:O showed a decline in PVYN-Wi but did not significantly affect the levels of PVYN:O (Figure 5E). Moreover, except for tubers doubly infected with PVYN-Wi and PVYNTN, all doubly infected tubers recorded a higher viral RNA load than singly infected tubers, as determined with universal primers (Supplementary Figure S5).
To determine whether interactions between PVY strains was influenced by the Ny gene, we investigated these interactions in tubers of ‘Russet Norkotah’, which does not contain the gene. Results showed that PVYN-Wi and PVYNTN, each acted synergistically with PVYO [40,41]. Strikingly, unlike observations in ‘Ranger Russet’, in ‘Russet Norkotah’, there were significantly lower levels of PVYNTN in leaves co-inoculated with PVYO. (Supplementary Figure S6). Thus, PVYO likely represses PVYNTN in the absence of the Ny gene, to which the host exhibits a HR in the presence of PVYO. Levels of PVYN:O were also repressed by PVYNTN and PVYN-Wi, but not by PVYO in ‘Russet Norkotah’ leaves but showed a significant increase in tubers co-infected with PVYNTN.
3.5. Effect of Temperature on PVY Cell-To-Cell Movement in Tubers
To determine whether low seed storage temperature influences PVY replication and cell-to-cell movement in potato tubers, we quantified the levels of PVYO and PVYNTN RNA over time in tubers at 4ºC, and at 22ºC, respectively. Samples were collected at 6, 24, and 48 hpi using a 0.5 cm corkborer at an approximate distance of 5 mm from the inoculation well, at position “a”, as well as at positions “b” and “c” for a total distance of approximately 1.5 cm from the center of the well, as illustrated in Figure 4A. Results of RT-qPCR quantification indicated that under both temperature conditions, viral RNA was detected at position “a” at 6 hpi, thus indicating that both PVY strains were replicating and carrying out cell-to-cell movement. Interestingly, viral RNA levels declined in samples collected from the same position over time, this may be due to tuber antiviral response.
There were differences between the levels of viral RNA in the two strains. For instance, PVYNTN RNA was significantly higher in ‘Desiree’ tubers at 4ºC compared with 22ºC from positions “a” through “c”. No decline was observed in ‘Ranger Russet’ at position “c” (Figure 6A). In ‘Eva’, there was almost no detectable viral RNA beyond position “a”, consistent with the fact that ‘Eva’ has PVY-extreme resistance, which likely involves virus movement. This result contrasts with the high levels of viral RNA observed along the infection gradient of tubers of the more susceptible ‘Desiree’ and ‘Ranger Russet’. Once again, the almost indetectable levels of viral RNA in ‘Eva’, compared with the less resistant cultivars is further evidence of viral replication and validates use of this approach to quantify virus replication in tubers. Consistent with results obtained in infectivity assessment above, we observed lower levels of PVYO in tubers of all three cultivars at both incubation temperatures compared with levels of PVYNTN, especially at 22ºC where ‘Eva’ and ‘Ranger Russet’ showed very low levels of PVYO RNA (Figure 6B). PVYO RNA levels were more uniform in ‘Desiree’ tubers at 22ºC.
3.6. Evidence of Virus Replication in Potato Tubers
To confirm that the viral RNA detected in this study was from a replicating virus, we carried out an RT-PCR targeting each of PVYNTN and PVYO Hc-Pro cistron negative sense strand RNA [(-)ssRNA], which unlike the (+)ssRNA is not packaged in the virion. Here, samples were collected at 6, 24, 48, and 96 hpi and total tuber RNA extracted as described in Material and Methods for reverse transcription of the (-)ssRNA of the Hc-Pro cistron. Reverse transcription was carried out using the Verso 1-step RT- PCR Reddymix kit (Thermo Scientific, USA) at 50ºC for 20 minutes with a primer targeting the (-)ssRNA of the Hc-Pro cistron. The reverse transcriptase was then inactivated for 10 minutes at 95ºC prior to PCR amplification of the cDNA. Two microliters of the reverse transcription reaction were utilized for PCR amplification using the Dreamtaq PCR mastermix kit (Thermo Scientific, USA) and both Hc-Pro forward and reverse primers (Table 1C). Agarose gel analysis of the PCR products showed the presence of an amplicon corresponding to Hc-Pro cistron (Supplementary Figure S7). Given that the inoculum used in this study was crude sap, where viral RNA is naturally degraded by RNAases, the (-)ssRNA obtained in this analysis could only have come from a replicating virus. This result, together with low levels of viral RNA recorded in cultivar ‘Eva’ as compared to RNA levels in the less resistant ‘Ranger Russet” and ‘Desiree’ observed above, indicates that the viral RNA detected in tubers in this study was from a replicating virus. Thus, the corkborer approach can be used to quantify replicating viral RNA in tubers.
4. Discussion
This study showed that the levels of PVYNTN RNA were consistently higher in potato tubers than those of PVYO. The results also showed that during co-infection, PVYNTN outcompeted PVYO in tubers of ‘Ranger Russet’ (Figure 6B) but not in those of ‘Russet Norkotah’. This is likely due to the presence in ‘Ranger Russet’ of Ny gene, which confers resistance to PVYO, but not to PVYNTN. Given that increasing importance of potato cultivars with the Ny gene in commercial potatoes [27,34,42], these interactions may, at least partly, explain the increasing importance in North America of PVYNTN, and to a lesser extent PVYN-Wi [3,14,15,26]. Further, although synergistic interactions are generally between unrelated viruses [43,44,45] and relationships between related viruses are mostly antagonistic (competitive) [43,44], these data show that synergistic interactions do occur in very closely related PVY strains and that these interactions are genotype and indeed tissue dependent. It is likely that there are host factors present in tubers with which proteins of either of the virus strains are interacting. Future studies will need to determine the mechanism of action, including by analyzing differentially expressed genes. Such a study will determine factors involved in PVYNTN induction of potato tuber necrotic ringspot disease (PTNRD).
It has been shown that Hc-Pro is the PVY avirulence factor corresponding to Nytbr and Ncspl [33]. The specific determinant was subsequently mapped to the Aspartic acid residue at position 419 (Asp419) in PVYO Hc-Pro [46]. In PVYN strains, there is Asp419 → Glu mutation. However, breakdown of Nytbr resistance in PVYNTN and PVYN-Wi was attributed to recombination rather than accumulation of point mutations [33]. The ability of PVYNTN and PVYN-Wi to overcome resistance conferred by the Ny gene may, at least partially, explain the antagonism between these strains and PVYO. Thus, PVYNTN and PVYN-Wi overcome the resistance conferred by Ncspl and outcompete PVYO. It must be stressed that necrosis alone may not be sufficient or essential for the restriction of viral movement. Indeed, other host factors have been reported to be involved in the recognition of the avirulence protein or signaling for HR, as well as light intensity and/or quality, influence the outcome of HR [28,29,30,31,32]. Hence, depending on the potato cultivar and environmental conditions, necrotic responses may vary phenotypically and, in addition to local necrosis, PVY may spread causing systemic necrosis and leaf-drop in the plant as observed on ‘Ranger Russet’ cultivar in this study.
Epidemiologically, these data support the hypothesis that the global increase in occurrence of PVYNTN, and to a limited extent, of PVYN-Wi, is at least partially due to the ability of these strains to outcompete PVYO, the hitherto predominant strain. Thus, in mixed infection situations, the fitness cost is more severe on PVYO than on PVYNTN or PVYN-Wi. Furthermore, it has been indicated that in potato cultivars with the Ny gene, such as ‘Ranger Russet’, severe systemic necrosis observed in this study, is beneficial in PVYO management because it leads to developmentally impaired plants, which are unlikely to be used for seed-tubers [2]. Given that a large proportion of cultivated potatoes have the Ny gene [8,27,42], this results in an increasing importance of PVYNTN and PVYN-Wi at the expense of PVYO. Moreover, the fact that there were no apparent viral symptoms in the foliage of potato plants infected by PVYNTN supports the view that much of the spread is likely through tubers. Equally epidemiologically important is the fact that potato plants doubly infected by PVYN-Wi and PVYO, and by PVYN:O and PVYO exhibited severe veinal and systemic necrosis, leading to plant death under our conditions. Thus, these plants are less likely to be used as seed tubers, thereby limiting these viral strains in mixed infected seed tubers.
Differences in fitness have been suggested to be the driving force in mixed infections between pathogens, and underpin the natural selection process [47,48]. For viruses, fitness is the extent to which the virus adapts to the host and can produce infectious progeny (i.e., replicative fitness) [44,49,50,51]. To be infectious, the virus must uncoat and release its genome, express its genes, and replicate. Nascent viral progeny must move cell-to-cell either as the naked genome or as encapsidated particles while evading host defenses [44,52]. The ability of PVYNTN and PVYN-Wi to outcompete PVYO can at least partially be explained by the ability of the Ny gene to interfere with at least one of these stages in the virus life cycle, thereby conferring resistance. This presumably favors the replication and/or spread of PVYNTN and PVYN-Wi.
Potato seed tubers are traditionally stored at low temperatures, usually between 3ºC and 5ºC [53], and then used in the next crop. Therefore, we investigated the effect of low storage temperature on the replication and cell-to-cell movement of PVYNTN and PVYO at 4ºC and 22ºC in tubers of cultivars ‘Desiree’, ‘Ranger Russet’, and ‘Eva’. Results showed similar levels of viral RNA at both temperature regimes. Thus, unlike the pathogenicity of tuber infecting bacterial and fungal diseases, which tend to decrease at low temperatures , PVY replication and cell-to-cell movement appear not to be affected by low storage temperatures. Epidemiologically, this indicates that even for low virus titer at harvest, PVY can continue to replicate and move cell-to-cell in the tuber during storage. It is important to note that others noted not substantial increase in PVY levels during storage [58]. Furthermore, the fact that the higher levels of PVYNTN over PVYO were more significant at 4ºC than at 22ºC indicates that PVYNTN is much fitter during seed tuber storage and may at least partially explain its gradual replacement of PVYO in North America, where seed tubers are traditionally stored at low temperatures.
One of the most limiting factors in studying virus pathogenicity in tubers is the difficulty of carrying out reproducible transmission in tubers. Thus, here, we developed a corkborer method and used it to infect tubers of all three potato cultivars efficiently and reproducibly.
Taken together, this study indicates that the current increase of PVYNTN and PVYN-Wi incidences in potato fields, especially in North America, may be due, at least partially, to their ability to antagonize and/or outcompete other strains, including especially the prevalent PVYO strain with the increasing incorporation of the Ny resistance gene into the potato cultivars since interaction between PVY strains is variety dependent [41].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Plant Biotic Interactions project number 2020-67018-31180.
Acknowledgements
We thank Joanna Friesner for the critical review of the manuscript.
References
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Molecular Plant Pathology 2011, 12, 938–954. [CrossRef]
- Karasev, A. V.; Gray, S.M. Continuous and Emerging Challenges of Potato Virus Y in Potato. Annual Review of Phytopathology 2013, 51, 571–586.
- Gray, S.; De Boer, S.; Lorenzen, J.; Karasev, A.; Whitworth, J.; Nolte, P.; Singh, R.; Boucher, A.; Xu, H. Potato Virus Y: An Evolving Concern for Potato Crops in the United States and Canada. Plant Dis 2010, 94, 1384–1397. [CrossRef]
- Karasev, A.V.; Hu, X.; Brown, C.J.; Kerlan, C.; Nikolaeva, O.V.; Crosslin, J.M.; Gray, S.M. Genetic Diversity of the Ordinary Strain of Potato Virus Y (PVY) and Origin of Recombinant PVY Strains. Phytopathology 2011, 101, 778–785. [CrossRef]
- Green, K.J.; Brown, C.J.; Gray, S.M.; Karasev, A.V. Phylogenetic Study of Recombinant Strains of Potato Virus Y. Virology 2017, 507, 40–52. [CrossRef]
- MacKenzie, T.D.B.; Nie, X.; Bisht, V.; Singh, M. Proliferation of Recombinant PVY Strains in Two Potato-Producing Regions of Canada, and Symptom Expression in 30 Important Potato Varieties with Different PVY Strains. Plant Dis 2019, 103, 2221–2230. [CrossRef]
- Hu, X.; Karasev, A.; … C.B.-J. of G.; 2009, undefined Sequence Characteristics of Potato Virus Y Recombinants. microbiologyresearch.org 2009, 90, 3033–3041. [CrossRef]
- Rowley, J.S.; Gray, S.M.; Karasev, A. V. Screening Potato Cultivars for New Sources of Resistance to Potato Virus Y. American Journal of Potato Research 2014 92:1 2015, 92, 38–48. [CrossRef]
- Carroll, J.E.; Smith, D.M.; Gray, S.M. Preferential Acquisition and Inoculation of PVYNTN over PVYO in Potato by the Green Peach Aphid Myzus Persicae (Sulzer). J Gen Virol 2016, 97, 797–802. [CrossRef]
- Boonham, N.; Walsh, K.; Preston, S.; North, J.; Smith, P.; Barker, I. The Detection of Tuber Necrotic Isolates of Potato Virus Y, and the Accurate Discrimination of PVY(O), PVY(N) and PVY(C) Strains Using RT-PCR. J Virol Methods 2002, 102, 103–112. [CrossRef]
- Nie, B.; Singh, M.; Murphy, A.; Sullivan, A.; Xie, C.; Nie, X. Response of Potato Cultivars to Five Isolates Belonging to Four Strains of Potato Virus Y. Plant Dis 2012, 96, 1422–1429. [CrossRef]
- Gebhardt, C.; Valkonen, J.P. Organization of Genes Controlling Disease Resistance in the Potato Genome. Annu Rev Phytopathol 2001, 39, 79–102. [CrossRef]
- Mestre, P.; Brigneti, G.; Baulcombe, D.C. An Ry-Mediated Resistance Response in Potato Requires the Intact Active Site of the NIa Proteinase from Potato Virus Y. Plant J. 2000, 23, 653–661.
- Dupuis, B.; Bragard, C.; Schumpp, O. Resistance of Potato Cultivars as a Determinant Factor of Potato Virus Y (PVY) Epidemiology. Potato Res. 2019, 62, 123–138. [CrossRef]
- Funke, C.N.; Nikolaeva, O.V.; Green, K.J.; Tran, L.T.; Chikh-Ali, M.; Quintero-Ferrer, A.; Cating, R.A.; Frost, K.E.; Hamm, P.B.; Olsen, N.; et al. Strain-Specific Resistance to Potato Virus Y (PVY) in Potato and Its Effect on the Relative Abundance of PVY Strains in Commercial Potato Fields. Plant Dis 2017, 101, 20–28. [CrossRef]
- Glais, L.; Tribodet, M.; Kerlan, C. Genomic Variability in Potato Potyvirus Y (PVY): Evidence That PVY(N)W and PVY(NTN) Variants Are Single to Multiple Recombinants between PVY(O) and PVY(N) Isolates. Arch Virol 2002, 147, 363–378. [CrossRef]
- Hu, X.; Karasev, A.V.; Brown, C.J.; Lorenzen, J.H. Sequence Characteristics of Potato Virus Y Recombinants. J Gen Virol 2009, 90, 3033–3041. [CrossRef]
- Lorenzen, J.; Nolte, P.; Martin, D.; Pasche, J.S.; Gudmestad, N.C. NE-11 Represents a New Strain Variant Class of Potato Virus Y. Arch Virol 2008, 153, 517–525. [CrossRef]
- Ogawa, T.; Nakagawa, A.; Hataya, T.; Ohshima, K. The Genetic Structure of Populations of Potato Virus Y in Japan; Based on the Analysis of 20 Full Genomic Sequences. Journal of Phytopathology 2012, 160, 661–673. [CrossRef]
- Jones, R. a. C. Strain Group Specific and Virus Specific Hypersensitive Reactions to Infection with Potyviruses in Potato Cultivars. Annals of Applied Biology 1990, 117, 93–105. [CrossRef]
- Mondal, S.; Lin, Y.-H.; Carroll, J.E.; Wenninger, E.J.; Bosque-Pérez, N.A.; Whitworth, J.L.; Hutchinson, P.; Eigenbrode, S.; Gray, S.M. Potato Virus Y Transmission Efficiency from Potato Infected with Single or Multiple Virus Strains. Phytopathology 2017, 107, 491–498. [CrossRef]
- Hühnlein, A.; Drechsler, N.; Steinbach, P.; Thieme, T.; Schubert, J. Comparison of Three Methods for the Detection of Potato Virus Y in Seed Potato Certification. Journal of Plant Diseases and Protection 2013, 120, 57–69.
- Taylor, S.; Wakem, M.; Dijkman, G.; Alsarraj, M.; Nguyen, M. A Practical Approach to RT-qPCR-Publishing Data That Conform to the MIQE Guidelines. Methods 2010, 50, S1-5. [CrossRef]
- Wilhelm, J.; Pingoud, A.; Hahn, M. Validation of an Algorithm for Automatic Quantification of Nucleic Acid Copy Numbers by Real-Time Polymerase Chain Reaction. Analytical Biochemistry 2003, 317, 218–225. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [CrossRef]
- Karasev, A.V.; Gray, S.M. Continuous and Emerging Challenges of Potato Virus Y in Potato. Annu Rev Phytopathol 2013, 51, 571–586. [CrossRef]
- Jones, R.A.C.; Vincent, S.J. Strain-Specific Hypersensitive and Extreme Resistance Phenotypes Elicited by Potato Virus Y Among 39 Potato Cultivars Released in Three World Regions Over a 117-Year Period. Plant Disease 2018, 102, 185–196. [CrossRef]
- Caplan, J.L.; Mamillapalli, P.; Burch-Smith, T.M.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplastic Protein NRIP1 Mediates Innate Immune Receptor Recognition of a Viral Effector. Cell 2008, 132, 449–462. [CrossRef]
- Tian, Y.-P.; Valkonen, J.P.T. Recombination of Strain O Segments to HCpro-Encoding Sequence of Strain N of Potato Virus Y Modulates Necrosis Induced in Tobacco and in Potatoes Carrying Resistance Genes Ny or Nc. Molecular Plant Pathology 2015, 16, 735–747. [CrossRef]
- Jin, H.; Liu, Y.; Yang, K.-Y.; Kim, C.Y.; Baker, B.; Zhang, S. Function of a Mitogen-Activated Protein Kinase Pathway in N Gene-Mediated Resistance in Tobacco. Plant J 2003, 33, 719–731. [CrossRef]
- Komatsu, K.; Hashimoto, M.; Ozeki, J.; Yamaji, Y.; Maejima, K.; Senshu, H.; Himeno, M.; Okano, Y.; Kagiwada, S.; Namba, S. Viral-Induced Systemic Necrosis in Plants Involves Both Programmed Cell Death and the Inhibition of Viral Multiplication, Which Are Regulated by Independent Pathways. Mol Plant Microbe Interact 2010, 23, 283–293. [CrossRef]
- Valkonen, J.P.; Rokka, V.M.; Watanabe, K.N. Examination of the Leaf-Drop Symptom of Virus-Infected Potato Using Anther Culture-Derived Haploids. Phytopathology 1998, 88, 1073–1077. [CrossRef]
- Moury, B.; Caromel, B.; Johansen, E.; Simon, V.; Chauvin, L.; Jacquot, E.; Kerlan, C.; Lefebvre, V. The Helper Component Proteinase Cistron of Potato Virus Y Induces Hypersensitivity and Resistance in Potato Genotypes Carrying Dominant Resistance Genes on Chromosome IV. Mol. Plant Microbe Interact. 2011, 24, 787–797. [CrossRef]
- Rowley, J.S.; Gray, S.M.; Karasev, A.V. Screening Potato Cultivars for New Sources of Resistance to Potato Virus Y. Am. J. Potato Res. 2015, 92, 38–48. [CrossRef]
- Plaisted, R.L.; Halseth, D.E.; Brodie, B.B.; Slack, S.A.; Sieczka, J.B.; Christ, B.J.; Paddock, K.M.; Peck, M.W. Eva:A Midseason Golden Nematode- and Virus-Resistant Variety for Use as Tablestock or Chipstock. Am. J. Pot Res 2001, 78, 65–68. [CrossRef]
- Nazarian-Firouzabadi, F.; Visser, R.G.F. Potato Starch Synthases: Functions and Relationships. Biochemistry and Biophysics Reports 2017, 10, 7–16. [CrossRef]
- Kumar, G.N.M.; Iyer, S.; Knowles, N.R. Extraction of RNA from Fresh, Frozen, and Lyophilized Tuber and Root Tissues. J Agric Food Chem 2007, 55, 1674–1678. [CrossRef]
- Vasanthan, T.; Bergthaller, W.; Driedger, D.; Yeung, J.; Sporns, P. Starch from Alberta Potatoes: Wet-Isolation and Some Physicochemical Properties. Food Research International 1999, 32, 355–365. [CrossRef]
- Wischmann, null; Hamborg Nielsen T, null; Lindberg Moller B, null In Vitro Biosynthesis of Phosphorylated Starch in Intact Potato Amyloplasts. Plant Physiol 1999, 119, 455–462. [CrossRef]
- Mondal, S.; Ghanim, M.; Roberts, A.; Gray, S.M. Different Potato Virus Y Strains Frequently Co-Localize in Single Epidermal Leaf Cells and in the Aphid Stylet. J Gen Virol 2021, 102. [CrossRef]
- Singh, M.; Singh, R.P. Host Dependent Cross-Protection between PVYN, PVY°, and PVA in Potato Cultivars and Solarium Brachycarpum. Canadian Journal of Plant Pathology 1995, 17, 82–86. [CrossRef]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Ny-1 and Ny-2 Genes Conferring Hypersensitive Response to Potato Virus Y (PVY) in Cultivated Potatoes: Mapping and Marker-Assisted Selection Validation for PVY Resistance in Potato Breeding. Mol Breed 2014, 34, 267–271. [CrossRef]
- Syller, J. Facilitative and Antagonistic Interactions between Plant Viruses in Mixed Infections. Mol Plant Pathol 2012, 13, 204–216. [CrossRef]
- Syller, J.; Grupa, A. Antagonistic Within-Host Interactions between Plant Viruses: Molecular Basis and Impact on Viral and Host Fitness. Mol Plant Pathol 2016, 17, 769–782. [CrossRef]
- Tatineni, S.; Riethoven, J.-J.M.; Graybosch, R.A.; French, R.; Mitra, A. Dynamics of Small RNA Profiles of Virus and Host Origin in Wheat Cultivars Synergistically Infected by Wheat Streak Mosaic Virus and Triticum Mosaic Virus: Virus Infection Caused a Drastic Shift in the Endogenous Small RNA Profile. PLoS ONE 2014, 9, e111577. [CrossRef]
- Glais, L.; Faurez, F.; Tribodet, M.; Boulard, F.; Jacquot, E. The Amino Acid 419 in HC-Pro Is Involved in the Ability of PVY Isolate N605 to Induce Necrotic Symptoms on Potato Tubers. Virus Res. 2015, 208, 110–119. [CrossRef]
- Abrams, M. Environmental Grain, Organism Fitness, and Type Fitness. Entangled Life 2014, 127–151. [CrossRef]
- Metcalf, C.J.E.; Birger, R.B.; Funk, S.; Kouyos, R.D.; Lloyd-Smith, J.O.; Jansen, V. a. A. Five Challenges in Evolution and Infectious Diseases. Epidemics 2015, 10, 40–44. [CrossRef]
- Barbour, J.D.; Grant, R.M. The Role of Viral Fitness in HIV Pathogenesis. Curr HIV/AIDS Rep 2005, 2, 29–34. [CrossRef]
- Domingo, E. Mechanisms of Viral Emergence. Vet Res 2010, 41, 38. [CrossRef]
- Wargo, A.R.; Kurath, G. Viral Fitness: Definitions, Measurement, and Current Insights. Curr Opin Virol 2012, 2, 538–545. [CrossRef]
- Elena, S.F.; Lalić, J. Plant RNA Virus Fitness Predictability: Contribution of Genetic and Environmental Factors. Plant Pathology 2013, 62, 10–18. [CrossRef]
- Milling, A.; Meng, F.; Denny, T.P.; Allen, C. Interactions with Hosts at Cool Temperatures, Not Cold Tolerance, Explain the Unique Epidemiology of Ralstonia Solanacearum Race 3 Biovar 2. Phytopathology 2009, 99, 1127–1134. [CrossRef]
- Gachango, E.; Hanson, L.E.; Rojas, A.; Hao, J.J.; Kirk, W.W. Fusarium Spp. Causing Dry Rot of Seed Potato Tubers in Michigan and Their Sensitivity to Fungicides. Plant Dis 2012, 96, 1767–1774. [CrossRef]
- Fiers, M.; Edel-Hermann, V.; Chatot, C.; Le Hingrat, Y.; Alabouvette, C.; Steinberg, C. Potato Soil-Borne Diseases. A Review. Agron. Sustain. Dev. 2012, 32, 93–132. [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M.K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato Dry Rot Disease: Current Status, Pathogenomics and Management. 3 Biotech 2020, 10, 503. [CrossRef]
- Magdalena, G.; Dariusz, M. Losses during Storage of Potato Varieties in Relation to Weather Conditions during the Vegetation Period and Temperatures during Long-Term Storage. Am. J. Potato Res. 2018, 95, 130–138. [CrossRef]
- Fox, A.; Evans, F.; Browning, I. Direct Tuber Testing for Potato Y Potyvirus by Real-Time RT-PCR and ELISA: Reliable Options for Post-Harvest Testing?*. EPPO Bulletin 2005, 35, 93–97. [CrossRef]
Figure 1.
Interactions between PVY strains PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato cultivar ‘Ranger Russet’. Systemically infected leaves of plants inoculated with PVYO displayed severe local veinal necrosis at 10 dpi while leaves of plants inoculated with PVYNTN, PVYN-Wi, and PVYN:O displayed necrotic spots (A). Leaves doubly infected with PVYN-Wi and PVYO, as well as with PVYN:O and PVYO displayed severe necrosis leading leaf death (B).
Figure 1.
Interactions between PVY strains PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato cultivar ‘Ranger Russet’. Systemically infected leaves of plants inoculated with PVYO displayed severe local veinal necrosis at 10 dpi while leaves of plants inoculated with PVYNTN, PVYN-Wi, and PVYN:O displayed necrotic spots (A). Leaves doubly infected with PVYN-Wi and PVYO, as well as with PVYN:O and PVYO displayed severe necrosis leading leaf death (B).

Figure 3.
Replication of four PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in tubers of three potato varieties with different levels of resistance to PVY. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. PVYNTN, and PVYN-Wi to a lesser extent, was observed to replicate to higher levels in ‘Desiree’ and ‘Ranger Russet’ but not in Eva. Mean differences were determined using a t-test.
Figure 3.
Replication of four PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in tubers of three potato varieties with different levels of resistance to PVY. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. PVYNTN, and PVYN-Wi to a lesser extent, was observed to replicate to higher levels in ‘Desiree’ and ‘Ranger Russet’ but not in Eva. Mean differences were determined using a t-test.

Figure 4.
Quantification of viral RNA in potato tubers at different times (hours) after inoculation. The inoculum was added to the central well using a 1 cm corkborer and samples collected at distances from the inoculum well using a 0.5 cm corkborer and samples collected from positions a, b, and c (4A) at 6, 24, 48, and 96 hours post inoculation. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. Tubers infected by PVYNTN and PVYO, displayed lower levels of viral RNA than tubers singly infected by either of the strains, as indicated by RT-qPCR analysis using strain-specific primers (4B and 4C). Mean differences were determined using a t-test.
Figure 4.
Quantification of viral RNA in potato tubers at different times (hours) after inoculation. The inoculum was added to the central well using a 1 cm corkborer and samples collected at distances from the inoculum well using a 0.5 cm corkborer and samples collected from positions a, b, and c (4A) at 6, 24, 48, and 96 hours post inoculation. Viral RNA levels were analyzed using RT-qPCR as described in Materials and Methods. Tubers infected by PVYNTN and PVYO, displayed lower levels of viral RNA than tubers singly infected by either of the strains, as indicated by RT-qPCR analysis using strain-specific primers (4B and 4C). Mean differences were determined using a t-test.

Figure 5.
Antagonism and synergistic interactions between PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato tubers. PVY strains were singly- and co-inoculated to determine the effect of mixed infection on replication, which was determined using RT-qPCR. Relative viral RNA levels were determined as described in Materials and Methods. PVYN-Wi was observed to repress PVYO and PVYNTN but not PVYN:O and the latter in turn represses PVYN-Wi as indicated by strain-specific primers. Mean differences were determined using a t-test.
Figure 5.
Antagonism and synergistic interactions between PVY strains, PVYO, PVYNTN, PVYN:O, and PVYN-Wi in potato tubers. PVY strains were singly- and co-inoculated to determine the effect of mixed infection on replication, which was determined using RT-qPCR. Relative viral RNA levels were determined as described in Materials and Methods. PVYN-Wi was observed to repress PVYO and PVYNTN but not PVYN:O and the latter in turn represses PVYN-Wi as indicated by strain-specific primers. Mean differences were determined using a t-test.

Figure 6.
Effect of temperature on tuber infectivity of PVYNTN (A) and PVYO (B). Tubers of ‘Desiree’, Eva, and ‘Ranger Russet’ were inoculated and incubated at 4ºC and 22ºC, respectively. Samples were collected 6 hpi, 24 hpi, and 48 hpi along the infection gradient from the periphery at ~22 mm (position “a”) from the inoculation well to ~1.5 cm (position “c”). Viral RNA levels were quantified using RT-qPCR as described in Materials and Methods.
Figure 6.
Effect of temperature on tuber infectivity of PVYNTN (A) and PVYO (B). Tubers of ‘Desiree’, Eva, and ‘Ranger Russet’ were inoculated and incubated at 4ºC and 22ºC, respectively. Samples were collected 6 hpi, 24 hpi, and 48 hpi along the infection gradient from the periphery at ~22 mm (position “a”) from the inoculation well to ~1.5 cm (position “c”). Viral RNA levels were quantified using RT-qPCR as described in Materials and Methods.

Table 1.
List of primers and targets used in this study.
| A/ RT-PCR | |||
|---|---|---|---|
| Target | Designation | Sequence (5’-3’) | Length (bp) |
| *PVYNTN |
NTN7350-F
NTN8266-R |
ACATCACCGATGAGCAGG
GTACATACCCTCGATTAGCA |
918 |
| *PVYO |
O1962-F
O2296-R |
TCAACATTCTATCCACCAAC
ACGTTTGAGTGTCATGGT |
335 |
| PVYN:O |
N:O1008-F
N:O1703-R |
GCACGTTCCAAGGTTACC
TCGCTTAGCATGATATTCCCT |
695 |
| **PVYN-Wi |
N-Wi_YN5-1780-F
N-Wi_YO3-2558-R |
TCCGAATGGGACAAGAAAACTTG
AGGCTCATCTAACAGCAACTGTC |
778 |
| B/ RT-qPCR*** | |||
| Target | Designation | Sequence (5’-3’) | Length (bp) |
| PVYNTN |
NTNq-6515-F NTNq-6631-R |
TCCGAGCTCCAGTGCAGAAT AAGTGCTGCCCGGTACATTG |
116 |
| PVYO |
Oq-4-F Oq-138-R |
CGCAAAAACACTCATAAAAGCTCA TGGTTGGAAGTGATGAAATTGCT |
134 |
| PVYN:O |
NOq-6444-F NOq-6574-R |
GGATATCATCCTCATCAAATGCCG TCGACGATGCATACTTCTCCTG |
130 |
| PVYN-Wi |
N-Wiq-35-F N-Wiq-156-R |
TTCCTTGCAATTCTCTTAAACGGT ACGAACCGAAACAGATTGTTGAC |
121 |
| All strains |
Uni-q-9426-F
Uni-q-9549-R |
GTGGCAGGGTGATTTCGTCA
AGAATCGCAACATCACCTAATCG |
123 |
| 1-alpha EF1α |
EF1-F EF1-R |
TGGAGGCACTCCCTGGTGACA
TGTGGCAGTCGAGCACTGGT |
194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Antagonism and Synergism Characterize the Interactions Between Four North American Potato Virus Y Strains
Vincent N Fondong
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








