Birthmarks Identificationandmx201205ryan
Birthmarks Identificationandmx201205ryan
Uploaded by
Danielcc LeeCopyright:
Available Formats
Birthmarks Identificationandmx201205ryan
Birthmarks Identificationandmx201205ryan
Uploaded by
Danielcc LeeCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Birthmarks Identificationandmx201205ryan
Birthmarks Identificationandmx201205ryan
Uploaded by
Danielcc LeeCopyright:
Available Formats
The first 3 months
Birthmarks
Emma Ryan Lachlan Warren
Identification and management
Background
Birthmarks are common in newborns, and their presence can cause much anxiety in new parents.
Objective
This article provides an update on common birthmarks and identifies those complex subtypes that may indicate potentially important associations or outcomes.
Birthmarks present at birth or soon after are a source of parental anxiety. This article focuses on common birthmarks seen by primary care physicians, helps identify patients requiring specific intervention, and explores recent developments in management.
Vascular birthmarks
When a patient presents with a vascular birthmark it is important to distinguish between a vascular tumour and a vascular malformation as this will predict outcomes and guide treatment. The Mulliken and Glowacki classification provides a framework to assist in differentiating vascular lesions (Table 1).1
Discussion
Birthmarks encompass a range of lesions presenting at birth or soon after. They can be divided into vascular, epidermal, pigmented and other subtypes. This article focuses on common birthmarks to help identify patients requiring specific intervention and explores recent developments in management. A minority of higher risk birthmarks have complications or systemic associations that need identification and further management. Birthmarks are common, and in most cases parents can be reassured they are only of cosmetic significance and that the appearance will improve over time.
Naevus simplex
Naevus simplex, colloquially known as salmon patch and stork mark, are pale-pink to bright-red capillary vascular malformations with indistinct borders that blanch and become more prominent with crying and straining. Naevus simplex most commonly affects the forehead, glabella, upper eyelids and nape; most spontaneously disappear between the ages of 1 and 3 years.2 Naevus simplex are thought to be related to immaturity of the vasculature and have a prevalence of 2060%. Treatment is usually not required.2
Keywords
naevus/congenital; haemangioma/congenital; skin neoplasms/ congenital; infant, newborn
Naevus flammeus (port wine stain)
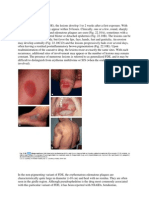
Naevus flammeus are capillary vascular malformations. They present as unilateral, homogenous red-to-violaceous macules involving the skin and, sometimes, the mucosa. They darken and thicken over time and have an incidence of 0.3%.3 Naevus flammeus can occur anywhere, but many involve the face where they follow the trigeminal nerve (fifth cranial nerve) distribution (branches 13 or V13)4 (Figure 1). While largely a cosmetic concern, a naevus flammeus on the face may be a marker for ophthalmologic or neurologic structural abnormalities and therefore further investigation is warranted.3 Sturge-Weber syndrome is defined as a facial naevus flammeus in association with an ipsilateral vascular malformation of the leptomeninges and eye.4 Location of the naevus helps identify newborns at risk of underlying abnormalities. If the opthalmic nerve (V1) territory is involved, an ophthalmological examination should be performed. If the V1 territory is associated with ophthalmologic or neurologic abnormalities, or if there is eyelid involvement or extension into the territories of maxillary (V2) and mandibular (V3) nerves, or if the naevus flammeus is bilateral, neuro-imaging should be organised with specialist
274 Reprinted from AUstralian FamilY PHYsiCian VOl. 41, NO. 5, MAY 2012
Table 1. Mulliken and Glowacki classication of vascular birthmarks1 Histology Presence at birth Course Female:male ratio Haemangioma Endothelial cell proliferation 40% are present at birth but usually appear as a small red mark Rapid postnatal growth and slow involution 3:1 Vascular malformation Normal endothelial cell turnover Present but not always clinically apparent Grows in proportion with the person throughout their life 1:1
review. Parents of the patient without V1 involvement may be reassured and informed about vascular laser as an option for treatment.3
Infantile haemangioma
Infantile haemangiomas (strawberry naevus) are benign vascular tumours. They occur in 510% of newborns, are more common in premature infants and have a female preponderance. Often unapparent at birth, they proliferate rapidly for several months, followed by gradual involution over several years.47 Superficial haemangiomas may be localised or segmental and appear as bright-red raised nodules (Figure 2). When located deeper in the dermis they appear as soft masses with a bluish tone. Segmental haemangiomas have a linear or geographic distribution and are more likely to have associated structural abnormalities.7 Most infantile haemangiomas involute without intervention and occupy uncomplicated sites. Those haemangiomas that are large or occupy the airway, eyes and vital organs can cause serious or life threatening complications and prompt specialist referral is required. Other complications include ulceration, secondary infection and scarring. Although resolution is considered complete in 76% of children by the age of 7 years, 50% will have minor residual changes such as telangiectasia or wrinkling.7 The treatment goal for infantile haemangioma is to prevent serious complications and permanent disfigurement (Figure 3). First line therapy has traditionally been systemic prednisolone during the proliferative phase at a dose of 2 mg/kg for 1216 weeks. Side effects included irritability, cushingoid appearance, adrenal suppression and susceptibility to infection.7 In 2008, propranolol was noted to shrink a haemangioma when used to manage a child with hypertrophic cardiomyopathy.5 Since then it has emerged as a relatively safe and effective treatment for infantile haemangioma.6 However, currently there is no clearly defined guideline for the use of propranolol for haemangiomas. Most groups have used 13 mg/kg/day in divided doses after baseline assessments.6 Our current recommendations include assessment for the presence of bronchospasm, cardiac disease and vascular anomalies by a dermatologist and a paediatrician. Baseline investigations should include a blood sugar level, blood pressure, electrocardiogram and clinical photography. Propranolol is commenced at 2 mg/kg/day in two divided doses for normal infants over 2 months of age. All patients should be closely monitored during the first dose for adverse cardiac and hypoglycaemic complications. This should be done in the hospital setting. Close follow
Figure 1. Naevus ammeus involving trigeminal nerve distribution V1 and V2
up by a paediatrician or paediatric dermatologist is recommended. Treatment is often required until the child is 1 year of age, with subsequent slow weaning of dosage.8 Other treatments include topical and intralesional steroids, interferon alpha, imiquimod, vincristine, cyclophosphamide and excision, however, there is weak evidence to support these alternative treatments. Pulse dye laser has been used with success, particularly in ulcerated haemangiomas.4
Epidermal naevi
Epidermal naevi are hamartomatous proliferations of the epithelium. They occur in one in 1000 live births with most appearing in the first year of life. Subtypes depend on the predominant cell type which include keratinocytes, the sebaceous gland, the pilosebaceous unit, and eccrine and apocrine glands.9 The naevi are composed of a localised clone of abnormal cells arising from a somatic mutation.4 Patients with unusual or distinctive keratinocytic naevi may need specialist assessment or genetic counselling.10 Naevus sebaceous (organoid naevus or naevus sebaceous of Jadassohn) are an epidermal naevus that present as well circumscribed plaques, which may be linear. Plaques are hairless, yellow and waxy and
Reprinted from AUstralian FamilY PHYsiCian VOl. 41, NO. 5, MAY 2012 275
FOCUS Birthmarks identification and management
The macular pigmentation of dermal melanocytosis has a blue-grey tone, usually involves less than 5% of the body, and is caused by sparse melanocytes residing in the mid to low dermis.9 Site-specific dermal melanocytosis includes the naevus of Ota a unilateral blue-brown facial patch often involving the ipsilateral sclera and occurring in dark-skinned people and the naevus of Ito, which involves the upper back and shoulder. Both lesions persist throughout life and patients should undergo skin and eye surveillance due to the [rare]
Figure 2. Ulcerated infantile haemangioma involving central upper lip before treatment
Figure 3. Infantile haemangioma involving central upper lip after treatment with prednisolone and propranolol for 4 months
Figure 4. Linear naevus sebaceous involving left temple
under the hormonal influences of puberty become more verrucous (wartlike). They appear most commonly on the scalp (Figure 4) but can present on the face and upper body. Benign and malignant tumours may arise in these naevi and a rapidly growing or ulcerated nodule within a sebaceous naevus should prompt excision. Previously, sebaceous naevi were excised in childhood to avoid the possibility of basal cell carcinoma development. As malignant progression in sebaceous naevi is rare, indications for surgery are controversial. Ongoing monitoring is a reasonable treatment option if there is no cosmetic impetus for surgery.4,10 Although epidermal naevi are most often sporadic, they can be part of a syndrome which includes neurologic, skeletal and ocular abnormalities, particularly if the naevus is extensive.4
Pigmented birthmarks
Dermal melanocytosis
Dermal melanocytosis (Mongolian spot) classically involves the lumbosacral area (Figure 5) and is seen at birth or soon after. It affects all races but has a higher prevalence in Asian children; most cases resolve in childhood.
Figure 5. Dermal melanocytosis (Mongolian spot) demonstrating classic distribution over the lumbosacral area
276 Reprinted from AUstralian FamilY PHYsiCian VOl. 41, NO. 5, MAY 2012
Birthmarks identification and management FOCUS
development of melanoma in these lesions. Naevus of Ota and naevus of Ito can be treated with pigment laser therapy.9
Congenital melanocytic naevus
Congenital melanocytic naevi (CMN) consist of proliferations of benign melanocytes. They are present at birth (Figure 6) with an incidence of 1%.9 Naevi are classified according to their projected adult size: small (<1.5 cm), medium (1.619.9 cm) or large (>20 cm). Common acquired naevi, although similar in appearance to small CMN, appear later in childhood. These naevi are often flat, with colour variability initially, and evolve over time to become thicker with hypertrichosis and colour change.11 This natural evolution presents challenges in monitoring. Progress photographs are a valuable management tool. The risk of developing melanoma in a CMN is significantly increased and there is a strong correlation with increasing size and the number of satellite lesions, with the highest risk occurring in large CMN >60 cm; these often develop in childhood.9,11 Patients can be reassured that the risk of melanomas in small and medium CMN is very low. Neurocutaneous melanosis is a rare syndrome characterised by large or multiple CMN and tumours of melanocytes within the CNS that may be asymptomatic or cause neurological symptoms. Magnetic resonance imaging (MRI) may be considered in the diagnosis of high risk patients with extensive melanocytic naevi.11 Management of CMN focuses on identifying complications and optimising cosmetic outcome.11 Infants with large CMN should be assessed in a multidisciplinary setting within the first weeks of life. Uncomplicated small CMN carry similar risks to acquired naevi and may be observed in the primary care setting. Treatment options include serial observation, excision, dermabrasion, curettage, hair and pigment reducing lasers and chemical peels.12
numbers.9 Solitary CALMs are common in up to 3% of healthy infants and 25% of children. Multiple CALMs are rare in healthy children and the majority of children with six or more CALMs will eventually be diagnosed with neurofibromatosis-1 (NF-1), potentially affecting multiple organ systems. Children with three or more CALMs should be monitored for other features of NF-1.13 Caf au lait macules may also be seen in multiple other rare syndromes.13 Isolated CALMs may be treated with pigment laser therapy.9
Summary
Birthmarks are common and in most cases parents can be reassured that they are only of cosmetic significance and that for many children the appearance will improve over time. A minority of higher risk birthmarks have complications or systemic associations that need identification and further management.
Authors
Emma Ryan MBBS, FRACGP, is a dermatology registrar, Royal Adelaide Hospital, Adelaide, South Australia. emma.ryan@internode.on.net Lachlan Warren BMBS, Dip(Obs), FRACOG, FRACGP, FACD, is Head of Unit, Department of Dermatology, Womens and Childrens Hospital, Adelaide, South Australia. Conflict of interest: none declared.
References
Caf au lait macules
Caf au lait macules (CALM) are well circumscribed light to darkbrown macules. The macules can occur anywhere on the body and are usually noted in infancy and childhood. Their colour is due to increased melanin in keratinocytes rather than an absolute increase in melanocyte
Figure 6. Large congenital melanocytic naevus with a truncal distribution and satellite naevi
1. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:41222. 2. Juern AM, Glick ZR, Drolet BA, et al. Nevus simplex: a reconsideration of nomenclature, sites of involvement, and disease associations. J Am Acad Dermatol 2010;63:80514. 3. Piram M, Lorette G, Sirinelli D, Herbreteau D, Giraudeau B, Maruani A. Sturge-Weber syndrome in patients with facial port-wine stain. Pediatr Dermatol 2012;29:327. 4. Moss C, Shahidullah H. Naevi and other developmental defects. In: Burns T, Breathnach SM, Cox, N and Griffiths C, editors. Rooks Textbook of Dermatology. Wiley-Blackwell: Oxford 2010; p.18. 5. Laut-Labrze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:264951. 6. Starkey E, Shahidullah H. Propranolol for infantile haemangiomas: a review. Arch Dis Child 2011;96:8903. 7. Schwartz RA, Sidor MI, Musumeci ML, Lin RL, Micali G. Infantile haemangiomas: a challenge in paediatric dermatology. J Eur Acad Dermatol Venereol 2010;24:6318. 8. Propranolol treatment of infantile haemangiomas. In: South Australian paediatric clinical guidelines. 2012. Available at www.health.sa.gov.au. 9. Barnhill RL, Rabinovitz H. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. Elsevier Ltd. 2008; p. 112. 10. Moody MN, Landau JM, Goldberg MD. Nevus sebaceous revisited. Pediatr Dermatol 2011;29:19. 11. Marghoob AA, Borrego JP, Halpern AC. Congenital melanocytic nevi: treatment modalities and management options. Semin Cutane Med Surg 2007;26:23140. 12. Kinsler VA, Birley J, Atherton DJ. Great Ormond Street Hospital for Children Registry for congenital melanocytic naevi: prospective study 1988-2007. Part 1 epidemiology, phenotype and outcomes. Br J Derm 2009;160:14350. 13. Nunley KS, Gao F, Albers AS, et al. Predictive value of caf au lait macules at initial consultation in the diagnosis of neurofibromatosis type 1. Arch Derm 2009;145:8837.
Reprinted from AUstralian FamilY PHYsiCian VOl. 41, NO. 5, MAY 2012 277
You might also like
- John Bradshaw - Cat Sense - How The New Feline Science Can Make You A Better Friend To Your Pet-Basic Books (2013) PDFDocument337 pagesJohn Bradshaw - Cat Sense - How The New Feline Science Can Make You A Better Friend To Your Pet-Basic Books (2013) PDFMelissa Fuenzalida88% (8)
- Wound Care Essentials 5th Edition 2020-538-669 - CompressedDocument132 pagesWound Care Essentials 5th Edition 2020-538-669 - CompressedRomana VayaniNo ratings yet
- Skin Manifestations of HIV DiseaseDocument31 pagesSkin Manifestations of HIV Diseaselovelots1234No ratings yet
- Table of Differentiation of ParasitesDocument18 pagesTable of Differentiation of ParasitesManuel RendonNo ratings yet
- Dermatology Questions and AnsDocument147 pagesDermatology Questions and AnsEl FaroukNo ratings yet
- Contact Dermatitis ModuleDocument68 pagesContact Dermatitis Moduletimvrghs123No ratings yet
- C U S T O M S: Boc Single Administrative DocumentDocument4 pagesC U S T O M S: Boc Single Administrative DocumentJohn LockeNo ratings yet
- Title: Figure 1. Acne VulgarisDocument7 pagesTitle: Figure 1. Acne VulgarisMelvin TiaraNo ratings yet
- Clinical Pearls DermatologíamatologyDocument4 pagesClinical Pearls DermatologíamatologyMaritza24No ratings yet
- Cards+Against+Paediatric+Dermatology+-+Vickie+Wells+&+Emma+Buxton+ (v1 0)Document146 pagesCards+Against+Paediatric+Dermatology+-+Vickie+Wells+&+Emma+Buxton+ (v1 0)Donna Ricotta100% (1)
- Berries in DermatologyDocument2 pagesBerries in DermatologyIjsrnet EditorialNo ratings yet
- Dermatology PDFDocument23 pagesDermatology PDFjonyNo ratings yet
- List of Dermatology Differential Diagnosis and Signs in DermatologyDocument54 pagesList of Dermatology Differential Diagnosis and Signs in DermatologyAhmadq76No ratings yet
- Introduction To Dermatology Assessment of A Dermatologic PatientDocument86 pagesIntroduction To Dermatology Assessment of A Dermatologic PatientAtif100% (1)
- Steroid Pulse Therapies in DermatologyDocument4 pagesSteroid Pulse Therapies in DermatologyWelly WijayantiNo ratings yet
- Clinico-Epidemiological Study of Dermatophytosis in Teaching Hospital of North KarnatakaDocument4 pagesClinico-Epidemiological Study of Dermatophytosis in Teaching Hospital of North KarnatakaAldo NovaNo ratings yet
- The Spectrum of Laser Skin Resurfacing Nonablative, FRACTIONAL and ABLATIVE LASER RESURFACINGDocument610 pagesThe Spectrum of Laser Skin Resurfacing Nonablative, FRACTIONAL and ABLATIVE LASER RESURFACINGBenazier Marcella BesmayaNo ratings yet
- Cutaneous Leishmaniasis: Caused by A Protozoa Called Leishmania PathogenesisDocument30 pagesCutaneous Leishmaniasis: Caused by A Protozoa Called Leishmania PathogenesisHudh HudNo ratings yet
- Fixed Drug Eruption, Bolognia DermatologyDocument2 pagesFixed Drug Eruption, Bolognia Dermatologyjacob89No ratings yet
- 323 (1) - Angry Back - Excited Skin SyndromeDocument6 pages323 (1) - Angry Back - Excited Skin Syndromecohbipc100% (1)
- Ex 2013 1 (Recurrent)Document30 pagesEx 2013 1 (Recurrent)alh basharNo ratings yet
- Melanocytic TumorsDocument254 pagesMelanocytic TumorsmixandgoNo ratings yet
- Contact DermatitisDocument70 pagesContact DermatitisThariq Mubaraq DrcNo ratings yet
- Dermatology NotesDocument37 pagesDermatology NotesTamilmani n.mNo ratings yet
- Dermatology and Venerology Notes 4Document111 pagesDermatology and Venerology Notes 4SpyrosNo ratings yet
- General Considerations For Topical PreparationsDocument6 pagesGeneral Considerations For Topical PreparationsOccamsRazorNo ratings yet
- Malignant Melanoma Research PaperDocument10 pagesMalignant Melanoma Research Paperapi-272931142No ratings yet
- AAD BF Paisley Tie DiagnosisDocument2 pagesAAD BF Paisley Tie Diagnosiskahkashanahmed065No ratings yet
- Dermatology Lectures JRRMMCDocument10 pagesDermatology Lectures JRRMMCGi Em100% (1)
- Mnemonics in DermatologyDocument2 pagesMnemonics in DermatologyShree ShresthaNo ratings yet
- Dermatology Biologics Boards Fodder DIR Winter 2017Document6 pagesDermatology Biologics Boards Fodder DIR Winter 2017riskhakov100% (1)
- Sqweqwesf Erwrewfsdfs Adasd Dhe: Dermnet NZDocument19 pagesSqweqwesf Erwrewfsdfs Adasd Dhe: Dermnet NZkdwazirNo ratings yet
- Han Book of Signs in Clinical DermatologyDocument180 pagesHan Book of Signs in Clinical DermatologyLumière Cosmetología integralNo ratings yet
- Connective Tissue DiseasesDocument54 pagesConnective Tissue DiseasesRatnakar Kamath100% (1)
- Dermatology Resident NotesDocument37 pagesDermatology Resident Notesedgar mandengNo ratings yet
- LALA Megatable DermaDocument39 pagesLALA Megatable DermaJorelle MarquezNo ratings yet
- Systemic Lupus Erythematosus SLEDocument7 pagesSystemic Lupus Erythematosus SLEDjdjjd SiisusNo ratings yet
- Topical Antibiotics in DermatologyDocument19 pagesTopical Antibiotics in DermatologyJejem Marandra EmkamasNo ratings yet
- Dermatologic Emergencies 2016Document116 pagesDermatologic Emergencies 2016saizadNo ratings yet
- Derma MegatableDocument21 pagesDerma MegatableNicole ChanNo ratings yet
- Dermatology Review NotesDocument8 pagesDermatology Review Notesnmb1986No ratings yet
- Andrews - Chapter 4 - Pruritus and Neurocutaneous DermatosesDocument40 pagesAndrews - Chapter 4 - Pruritus and Neurocutaneous DermatosesAngeli Ramos-EstrellaNo ratings yet
- An Approach To The Patient With ErythrodermaDocument42 pagesAn Approach To The Patient With ErythrodermaShakilNo ratings yet
- Srinadh Neonatal Skin Conditions FinalDocument110 pagesSrinadh Neonatal Skin Conditions FinalSrinadh Pragada100% (1)
- Dermatology Mcqs For PGDocument204 pagesDermatology Mcqs For PGjusta7863No ratings yet
- Fungal Skin Infections-1Document27 pagesFungal Skin Infections-1Fabb Nelson100% (1)
- Dermatology: Minci YazuminDocument48 pagesDermatology: Minci Yazuminminci senseiNo ratings yet
- Panniculitis 2021Document45 pagesPanniculitis 2021karimahihdaNo ratings yet
- Dermatology Slides - Introduction To Clinical DermatologyDocument34 pagesDermatology Slides - Introduction To Clinical DermatologyAzry Mustapa100% (1)
- Technology in Practical Dermatology: Non-Invasive Imaging, Lasers and Ulcer ManagementFrom EverandTechnology in Practical Dermatology: Non-Invasive Imaging, Lasers and Ulcer ManagementMichele FimianiNo ratings yet
- DA Sistemik TerapiDocument11 pagesDA Sistemik TerapiariyatiNo ratings yet
- Resident Handbook 2008Document31 pagesResident Handbook 2008Vasilis ImvriotisNo ratings yet
- Cutaneous Lupus Erythematosus: SGD B3Document41 pagesCutaneous Lupus Erythematosus: SGD B3Che Haniff100% (3)
- Dermatology Approach: Fayza RayesDocument108 pagesDermatology Approach: Fayza RayesMinghan Shi100% (2)
- Skintox Practiceguidelines EGFR SCCDocument17 pagesSkintox Practiceguidelines EGFR SCCFelipe Scholz RamosNo ratings yet
- ErytrasmaDocument17 pagesErytrasmaAndrean LinataNo ratings yet
- Infectious DermatologyDocument206 pagesInfectious DermatologyAaron Christian Earl VillosoNo ratings yet
- Ghid Anestezie Locala PDFDocument19 pagesGhid Anestezie Locala PDFanaNo ratings yet
- Final MCQs For The 4th Yaer StudentsDocument49 pagesFinal MCQs For The 4th Yaer StudentseidNo ratings yet
- Vitiligo: Vishnu Narayanan M.RDocument59 pagesVitiligo: Vishnu Narayanan M.RMani ManiNo ratings yet
- Leishmaniasis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandLeishmaniasis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Diagnosis of Iron Deficiency and Iron Overload Nov 06Document8 pagesDiagnosis of Iron Deficiency and Iron Overload Nov 06Danielcc LeeNo ratings yet
- Commonrashes in Neonates201205suDocument6 pagesCommonrashes in Neonates201205suDanielcc LeeNo ratings yet
- 14 Essentials To Assessment and Care PlanMT2013!08!018-BRODATY - 0Document9 pages14 Essentials To Assessment and Care PlanMT2013!08!018-BRODATY - 0Danielcc Lee100% (1)
- Full Blood Count Apr04, DR Eva RaikDocument7 pagesFull Blood Count Apr04, DR Eva RaikDanielcc LeeNo ratings yet
- 14 Essentials To MxMT2013!09!029-BRODATY - 0Document11 pages14 Essentials To MxMT2013!09!029-BRODATY - 0Danielcc LeeNo ratings yet
- Diabetes Diagnosis and Monitoring, April 2002 Author Peter ColmanDocument13 pagesDiabetes Diagnosis and Monitoring, April 2002 Author Peter ColmanDanielcc LeeNo ratings yet
- Interpretation of The eGFR Jul 07Document8 pagesInterpretation of The eGFR Jul 07Danielcc LeeNo ratings yet
- Avoiding Unnecessary Laboratory Tests May 2008Document8 pagesAvoiding Unnecessary Laboratory Tests May 2008Danielcc LeeNo ratings yet
- Bed Bugs: What The GP Needs To KnowDocument5 pagesBed Bugs: What The GP Needs To KnowDanielcc LeeNo ratings yet
- Blood Transfusion ProcedureDocument0 pagesBlood Transfusion ProcedureDanielcc LeeNo ratings yet
- GeriatricsDocument12 pagesGeriatricsDanielcc Lee100% (1)
- Elevated Ferritin What Should GP KnowDocument5 pagesElevated Ferritin What Should GP KnowDanielcc LeeNo ratings yet
- Drug InteractionDocument4 pagesDrug InteractionDanielcc LeeNo ratings yet
- Ms 96 03Document104 pagesMs 96 03Saadatul AliyahNo ratings yet
- PMD55 Manual de Diferencial de Presion de EndresDocument48 pagesPMD55 Manual de Diferencial de Presion de EndresGuillermoAlejandroCajalNo ratings yet
- Operations Management Chapter 7Document51 pagesOperations Management Chapter 7Jerome 高自立 KoNo ratings yet
- Sentence ErrorDocument52 pagesSentence ErrorJąhąnząib Khąn KąkąrNo ratings yet
- Chapter 03Document61 pagesChapter 03JohnJaye100% (7)
- E-Rapor Kurikulum Merdeka SMP - Aplikasi E-Rapor Pelaksana Kurikulum Merdeka Jenjang SMPDocument3 pagesE-Rapor Kurikulum Merdeka SMP - Aplikasi E-Rapor Pelaksana Kurikulum Merdeka Jenjang SMPNINDI LIONITANo ratings yet
- 2001 03 12 Acute MonoarthritisDocument14 pages2001 03 12 Acute MonoarthritisZH. omg sarNo ratings yet
- Internship Reflection PaperDocument3 pagesInternship Reflection Paperapi-483655169No ratings yet
- UB Internship Report UpdateDocument55 pagesUB Internship Report UpdatepaulaNo ratings yet
- Futurlux Head BeamDocument8 pagesFuturlux Head BeamSouca Paul IoanNo ratings yet
- Product KeysDocument2 pagesProduct KeysHorizon 99100% (1)
- GDB Rocks!Document91 pagesGDB Rocks!openid_FyIGs9jYNo ratings yet
- Lean Blow Out Limits of A Gas Turbine CombustorDocument10 pagesLean Blow Out Limits of A Gas Turbine CombustorFayçal MahieddineNo ratings yet
- SAFETY Action Plan For 2016Document6 pagesSAFETY Action Plan For 2016naguNo ratings yet
- 3071 Standard-Range: Used SymbolsDocument3 pages3071 Standard-Range: Used Symbolsgerardo castilloNo ratings yet
- WM Process CycleDocument39 pagesWM Process CycleAnil KumarNo ratings yet
- School Prospectus Parkside Primary School 2023 24Document35 pagesSchool Prospectus Parkside Primary School 2023 24H. Aadi TVNo ratings yet
- Common AccidentDocument11 pagesCommon AccidentReleyNo ratings yet
- Case Study - DysonDocument5 pagesCase Study - DysonTrần Khánh ĐoanNo ratings yet
- Text and Visual Dimensions Oftext and Visual Dimensions of MEDIA AND INFORMATIONDocument42 pagesText and Visual Dimensions Oftext and Visual Dimensions of MEDIA AND INFORMATIONVinnie GognittiNo ratings yet
- Individual SportDocument360 pagesIndividual SportMarvin MagararuNo ratings yet
- Step-By-Step DIY Guide: To Salesforce ForDocument84 pagesStep-By-Step DIY Guide: To Salesforce Formohemedsk100% (2)
- A Presentation On Dham Aparadha /dham Seva: Prepared by Akash Uniyal, Deepak and ChetanDocument14 pagesA Presentation On Dham Aparadha /dham Seva: Prepared by Akash Uniyal, Deepak and ChetanSyllabus HelpNo ratings yet
- Table of Roman Equivalents of Greek GodsDocument4 pagesTable of Roman Equivalents of Greek GodsToqeer Raza100% (1)
- Centrifugal Pump Impeller Vane ProfileDocument32 pagesCentrifugal Pump Impeller Vane Profiletomsiri100% (1)
- Sartorius 2020 PD Particle Validation Standards SoloHill Particle Validation StandardsDocument4 pagesSartorius 2020 PD Particle Validation Standards SoloHill Particle Validation StandardsbioNo ratings yet
- Automechanika PSR 2007Document4 pagesAutomechanika PSR 2007Donald GirsangNo ratings yet
- 3rd-7e's - LOCATING PLACESDocument3 pages3rd-7e's - LOCATING PLACESRod ReyesNo ratings yet