0 ratings0% found this document useful (0 votes)
251 views3.3 - Phase Changes
3.3 - Phase Changes
Uploaded by
popadoraPhase changes involve a reversible physical change between different states of matter such as melting, freezing, vaporization, and condensation. These changes occur at consistent temperatures and involve the absorption or release of energy. During phase changes, the temperature of the substance remains constant while large amounts of energy are used to break or form molecular bonds as molecules rearrange their structure. The energy required or released during phase changes depends on the type of change and properties of the substance.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as RTF, PDF, TXT or read online from Scribd
3.3 - Phase Changes
3.3 - Phase Changes
Uploaded by
popadora0 ratings0% found this document useful (0 votes)
251 views3 pagesPhase changes involve a reversible physical change between different states of matter such as melting, freezing, vaporization, and condensation. These changes occur at consistent temperatures and involve the absorption or release of energy. During phase changes, the temperature of the substance remains constant while large amounts of energy are used to break or form molecular bonds as molecules rearrange their structure. The energy required or released during phase changes depends on the type of change and properties of the substance.
Original Description:
3.3 - Phase Changes
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
RTF, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Phase changes involve a reversible physical change between different states of matter such as melting, freezing, vaporization, and condensation. These changes occur at consistent temperatures and involve the absorption or release of energy. During phase changes, the temperature of the substance remains constant while large amounts of energy are used to break or form molecular bonds as molecules rearrange their structure. The energy required or released during phase changes depends on the type of change and properties of the substance.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as RTF, PDF, TXT or read online from Scribd
Download as rtf, pdf, or txt
0 ratings0% found this document useful (0 votes)
251 views3 pages3.3 - Phase Changes
3.3 - Phase Changes
Uploaded by
popadoraPhase changes involve a reversible physical change between different states of matter such as melting, freezing, vaporization, and condensation. These changes occur at consistent temperatures and involve the absorption or release of energy. During phase changes, the temperature of the substance remains constant while large amounts of energy are used to break or form molecular bonds as molecules rearrange their structure. The energy required or released during phase changes depends on the type of change and properties of the substance.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as RTF, PDF, TXT or read online from Scribd
Download as rtf, pdf, or txt
You are on page 1of 3
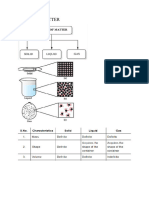
Phase Changes
Characteristics of Phase changes:
phase change is a reversible physical change between states of matter - melting, freezing, vaporization, condensation, sublimation, and deposition, with characteristics related to energy and temperatured >Temperature and Phase Changes-temperature of substance doesn't change during a phase change naphthalene = mothball compound - solid->liquid = up to 8 C, then stops until change is complete - l->s = temp drops to 8 C and stays until freezing is complete - l->g = temp rises to !"8 C until boiling is complete >#nergy and Phase Changes phase change = energy transferred btwn substance and surrounding, w/ direction deperding on type of change - energy is absorbed$released during phase change endothermic changes = system absorbs energy; melting, etc heat of fusion = !oules "##$ for water% water releases ##$ ! during freezing and absorbs ame amount during freezing - so, farmers spray water over cold crops b/c water gives of heat during freezing - another e&le = ice ring with different layers of insulation, etc e%othermic change = releases energy, including freezing
&elting and 'reezing(
- water molecule arrangements become less orderly during melting and more orderly during freezing) >&eltingice/solids = attractions btwn molecules 'eep them fi&ed more heat = more average 'inetic energy of motion
at melting point "( ) for water%, particles have enough energy to brea' bonds and move, and melting is complete any energy gain later = more temp and ave 'inetic energy >'reezing cools down so the ave 'inetic energy decreases and the particles move slowly enough so the bonds have an effect and the molecules are in an organized bond isn*t always cold "eg silicon%
*aporization and Condensation(
vaporization = change from liquid to gas "endothermic, must absorb energy% heat of vaporization +varies, = energy a liquid substance must gain to become a gas water = +,+,- !oules gained per gram at vaporization at .(( ) ! types of vaporization( evaporation at surface, below the boiling point >#vaporation( evaporation is vaporization at surface, w/ temp below boiling point molecules at surface gain energy and become vapor in a closed container, vapor collects above the liquid and causes vapor pressure between walls and collisions of vapor pressure increases with temp - higher temps = more particles that are free to overcome attractions >-oiling( heating water: temp increases and vapor pressure increases to atmospheric pressure, when it boils: the boiling point of water is " C / boiling point, molecules below surface have enough energy to overcome bonds, so they become bubbles, float to surface, and are realeased into the air depends on atmostpheric pressure - water / sea level = .(( ) - higher elevations = lower >Condensation( condensation = gas/vapor -> liquid "e&othermic%
.ublimation and /eposition(
sublimation = solid to gas directly without liquid state, endothermic process - dry ice = condensed )0+ which causes clouds during sublimation deposition = opposite of sublimation, from gas + solid, causes frost and is e&othermic
You might also like
- Design of Steel Frames Using SAP2000 - Illustrative ExamplesDocument36 pagesDesign of Steel Frames Using SAP2000 - Illustrative ExamplesvtalexNo ratings yet
- Y 7 IYub 8 Mrqir Qupg SQ0 TDocument47 pagesY 7 IYub 8 Mrqir Qupg SQ0 TG124Rohit MajiNo ratings yet
- FKCH 8 HHM BYSJxe F8 ZG 74Document45 pagesFKCH 8 HHM BYSJxe F8 ZG 74MahaNo ratings yet
- Calorimetry 2023Document10 pagesCalorimetry 2023Yatharth TiwariNo ratings yet
- Effect of Temperature in The Change of State of Matters PDFDocument4 pagesEffect of Temperature in The Change of State of Matters PDFSourya AichNo ratings yet
- Specific Heat CapacityDocument12 pagesSpecific Heat Capacitytanvir.md.ahsanNo ratings yet
- FT Physics NotesDocument13 pagesFT Physics Notes410230675No ratings yet
- Physics - Heat Definiton of Terms: 1. Internal EnergyDocument8 pagesPhysics - Heat Definiton of Terms: 1. Internal EnergyRaistlin Chan Ching Kit0% (1)
- 1.Dr - Ahmed Samy - PhysicsDocument21 pages1.Dr - Ahmed Samy - PhysicsKhaled AhmedNo ratings yet
- Concept of Latent Heat. BaseDocument18 pagesConcept of Latent Heat. BaseHarsh TripathiNo ratings yet
- Change in The F-WPS OfficeDocument3 pagesChange in The F-WPS OfficekimkenzjiaNo ratings yet
- HeatDocument7 pagesHeatkrushnakadam0029No ratings yet
- Heat Notes 2024Document5 pagesHeat Notes 2024saesha2801No ratings yet
- Matter In Our Surroundings 2021-2022Document14 pagesMatter In Our Surroundings 2021-2022spotlightstar3243No ratings yet
- Specialized Training For Oil Tankers CH 2 Basic Properties of Petroleum and Its HazardsDocument36 pagesSpecialized Training For Oil Tankers CH 2 Basic Properties of Petroleum and Its HazardsFairuz TotoqNo ratings yet
- 240816070841131496calorimetry 2024Document13 pages240816070841131496calorimetry 2024sainimanjeet5681No ratings yet
- Thermal Physics PartDocument17 pagesThermal Physics PartSammyJayNo ratings yet
- Notes On CHP 1Document7 pagesNotes On CHP 1shravanisantoshraneNo ratings yet
- Heat Transfer 1Document32 pagesHeat Transfer 1Fallen AngelNo ratings yet
- Unit: 1 Refrigeration System and Refrigeration EquipmentsDocument13 pagesUnit: 1 Refrigeration System and Refrigeration EquipmentsrajasekarNo ratings yet
- Second: Heat Transfer Correlations For Flow Boiling Heat TransferDocument9 pagesSecond: Heat Transfer Correlations For Flow Boiling Heat Transfermalek mustafaNo ratings yet
- Temperature: Motion Average Kinetic Energy Warmth CoolnessDocument22 pagesTemperature: Motion Average Kinetic Energy Warmth CoolnessHarsh BhatiaNo ratings yet
- BMS Group 1Document32 pagesBMS Group 1sandaliNo ratings yet
- Air Conditioning: Basic Refrigeration Principles: Unit-1Document19 pagesAir Conditioning: Basic Refrigeration Principles: Unit-1Mr. Ahamed Fazeel Akram B.ArchNo ratings yet
- Basicsofsteamboilers Sectionb 161124193357Document42 pagesBasicsofsteamboilers Sectionb 161124193357Emmanuel AyisiNo ratings yet
- (AA) - What Is Steam MARINE STEAM SYSTEMDocument65 pages(AA) - What Is Steam MARINE STEAM SYSTEMrajpratik1561No ratings yet
- Parte 1 Taller Transferencia de CalorDocument2 pagesParte 1 Taller Transferencia de Calorsebastian buitragoNo ratings yet
- What Is SteamDocument12 pagesWhat Is SteamZeljko CisarNo ratings yet
- Air Conditioning: Basic Refrigeration Principles: Unit-1Document16 pagesAir Conditioning: Basic Refrigeration Principles: Unit-1Prabha Karen PalanisamyNo ratings yet
- Moisture Clouds and PrecipitationDocument36 pagesMoisture Clouds and PrecipitationA.j. SanchezNo ratings yet
- Warming The Earth and The AtmosphereDocument46 pagesWarming The Earth and The AtmosphereKylene AlimNo ratings yet
- Heat TransferDocument11 pagesHeat Transferarijitmandal4578No ratings yet
- S5 Physics Revision NotesDocument40 pagesS5 Physics Revision NotesLibertyTong100% (1)
- 2 States of Matter NotesDocument14 pages2 States of Matter Notesafnan.6556No ratings yet
- Heating and Cooling Curve of A Substance 1Document5 pagesHeating and Cooling Curve of A Substance 1bennaorbino272006No ratings yet
- Warming The Earth and The AtmosphereDocument46 pagesWarming The Earth and The AtmosphereSalih TrexieNo ratings yet
- Changes in The States of MatterDocument8 pagesChanges in The States of MatterNisha JodhanNo ratings yet
- PHASE CHANGE Hand OutDocument8 pagesPHASE CHANGE Hand Outjoel rosalNo ratings yet
- 0a - RDG - Heat and Calorimetry - ReviewDocument8 pages0a - RDG - Heat and Calorimetry - ReviewIgnacio OreiroNo ratings yet
- Phase Changes Group 6Document8 pagesPhase Changes Group 6caezzar sangcupanNo ratings yet
- Heat TransferDocument11 pagesHeat TransferArnab HazraNo ratings yet
- ETD Chapter 3Document17 pagesETD Chapter 3Vasantha SeelanNo ratings yet
- Melting Boiling and EvaporationDocument19 pagesMelting Boiling and EvaporationAnmol MalgotraNo ratings yet
- The Principles of a Combined Cycle Power PlantDocument47 pagesThe Principles of a Combined Cycle Power PlantHOJJAT BAGHERINo ratings yet
- unit-1AIR CONDITIONING Basic PrinciplesDocument14 pagesunit-1AIR CONDITIONING Basic PrinciplesRITHANYAANo ratings yet
- TD MODULE 5Document30 pagesTD MODULE 5mujeebNo ratings yet
- THERMAL EFFECTS Part 3Document10 pagesTHERMAL EFFECTS Part 3itsshaunboteNo ratings yet
- Essay On ConductionDocument4 pagesEssay On Conductionnzila43No ratings yet
- All Document Reader 1730210054728Document45 pagesAll Document Reader 1730210054728mbbspharmdNo ratings yet
- State ChangesDocument4 pagesState ChangesSaanvi LambaNo ratings yet
- Heat TransferDocument13 pagesHeat Transferarijitmandal4578No ratings yet
- Saturated Steam Vs Supereated SteamDocument3 pagesSaturated Steam Vs Supereated SteamPravin KumarNo ratings yet
- 9 Science MatterDocument6 pages9 Science MatterAjay AnandNo ratings yet
- Chem M3 PDFDocument7 pagesChem M3 PDFZarylle De AsasNo ratings yet
- Lab Report 2Document9 pagesLab Report 2Samsung Note 9No ratings yet
- SDSDSDDocument1 pageSDSDSDMartillano, Oliver C.No ratings yet
- Conduction, Convection and Sensible and Latent HeatDocument8 pagesConduction, Convection and Sensible and Latent HeatAbhinav MahawarNo ratings yet
- STUDY MATERIAL CLASS-9TH, SCIENCE 2023-24-outputDocument86 pagesSTUDY MATERIAL CLASS-9TH, SCIENCE 2023-24-outputAbrar PanjNo ratings yet
- TP6 Thermal TransferDocument7 pagesTP6 Thermal TransfernonofotlhoweNo ratings yet
- Scout Scout Jem Jem Dill DillDocument2 pagesScout Scout Jem Jem Dill DillpopadoraNo ratings yet
- African Women EntrepreneursDocument3 pagesAfrican Women EntrepreneurspopadoraNo ratings yet
- Shri,,, Ra,,, ,,ma, Dra,,, Sugunasa Aan,: S,,, SNDM GGS, S,,, MGM, NsgsDocument2 pagesShri,,, Ra,,, ,,ma, Dra,,, Sugunasa Aan,: S,,, SNDM GGS, S,,, MGM, NsgspopadoraNo ratings yet
- Mohana Swarajathi ScriptDocument4 pagesMohana Swarajathi Scriptpopadora0% (1)
- Rough DraftDocument5 pagesRough DraftpopadoraNo ratings yet
- Humphrey Harold Harrington: Blank, Bewildered, BummedDocument3 pagesHumphrey Harold Harrington: Blank, Bewildered, BummedpopadoraNo ratings yet
- Dispersion, and IonizationDocument2 pagesDispersion, and IonizationpopadoraNo ratings yet
- Unfiled NotesDocument82 pagesUnfiled NotespopadoraNo ratings yet
- 07 PolygonsDocument34 pages07 PolygonspopadoraNo ratings yet
- 2 B - Plane Theorems DAY 1 TeacherDocument13 pages2 B - Plane Theorems DAY 1 TeacherpopadoraNo ratings yet
- Find Every Measure You Can: TaiatrrnDocument34 pagesFind Every Measure You Can: TaiatrrnpopadoraNo ratings yet
- Electroplating Solder Blue and Green Television PhosphorsDocument1 pageElectroplating Solder Blue and Green Television PhosphorspopadoraNo ratings yet
- 1804 - Oliver Evan's First Steam Boat 1807-First Railroad Service With HorsesDocument3 pages1804 - Oliver Evan's First Steam Boat 1807-First Railroad Service With HorsespopadoraNo ratings yet
- Chapter 2 PowerPointDocument38 pagesChapter 2 PowerPointpopadora100% (1)
- Chapter 13 Forces in Fluids Study GuideDocument2 pagesChapter 13 Forces in Fluids Study GuidepopadoraNo ratings yet
- Unit 6: Volcanoes Study Guide: Questions/Main Ideas NotesDocument2 pagesUnit 6: Volcanoes Study Guide: Questions/Main Ideas NotespopadoraNo ratings yet
- Chapter 14 and 15 History NotesDocument23 pagesChapter 14 and 15 History NotespopadoraNo ratings yet
- Torque Control in Field Weakening ModeDocument84 pagesTorque Control in Field Weakening Modecl108No ratings yet
- Selection of Cables/ConductorsDocument37 pagesSelection of Cables/ConductorsshuwingNo ratings yet
- Revised - NSEJS 2019-20 (17 Nov 2019) - Answers & SolutionsDocument22 pagesRevised - NSEJS 2019-20 (17 Nov 2019) - Answers & SolutionsKousika VijayakumarNo ratings yet
- (Book Solution) 6-2, Mechanics of Materials by R C Hibbeler) - Draw The Shear and Moment Diagrams For The Simply Supported BeamDocument1 page(Book Solution) 6-2, Mechanics of Materials by R C Hibbeler) - Draw The Shear and Moment Diagrams For The Simply Supported BeamGuilherme RamosNo ratings yet
- C12 (11-Bis) 18.12.2008 24 SlidesDocument24 pagesC12 (11-Bis) 18.12.2008 24 SlidesHazzy JoshuaNo ratings yet
- Dewey Decimal Classification in Myanmar (Official)Document63 pagesDewey Decimal Classification in Myanmar (Official)Aye Khaing100% (1)
- Lecture Notes - Oscillations and WavesDocument55 pagesLecture Notes - Oscillations and WavesRashmikaNo ratings yet
- Sea Saw Gen FinalDocument27 pagesSea Saw Gen FinalSanjay Jadhav67% (3)
- Instant download Nematic and cholesteric liquid crystals concepts and physical properties illustrated by experiments 1st Edition Patrick Oswald pdf all chapterDocument85 pagesInstant download Nematic and cholesteric liquid crystals concepts and physical properties illustrated by experiments 1st Edition Patrick Oswald pdf all chaptermarlydembyij100% (7)
- 4.newton's Law of MotionDocument29 pages4.newton's Law of MotionHo Fung ChowNo ratings yet
- Notes For Chapter 20Document28 pagesNotes For Chapter 20edsoneiccNo ratings yet
- Welded Moment Connection PDFDocument22 pagesWelded Moment Connection PDFRajasekar MeghanadhNo ratings yet
- Li 2018Document19 pagesLi 2018Eduardo SaavedraNo ratings yet
- CBSE Class 11 Physics Sample Paper Set 5Document3 pagesCBSE Class 11 Physics Sample Paper Set 5Prajin MuruganNo ratings yet
- NotesDocument390 pagesNotesAshiq AliNo ratings yet
- Chapter 2: The Chemical Context of LifeDocument40 pagesChapter 2: The Chemical Context of LifeDannyNo ratings yet
- 7G Particle Theory: 3 0 Stage DDocument3 pages7G Particle Theory: 3 0 Stage DBen PassmoreNo ratings yet
- Module 6Document14 pagesModule 6daanNo ratings yet
- TutorialPDE2010 1Document3 pagesTutorialPDE2010 1Ong Teck ChongNo ratings yet
- Aisi Example II-7Document24 pagesAisi Example II-7LauraMilenaHernándezTorresNo ratings yet
- Design and Analysis of Army Vehicle Chassis: Abstract-In The Current Project Manual DesignDocument8 pagesDesign and Analysis of Army Vehicle Chassis: Abstract-In The Current Project Manual DesignRAMESH NARAYANANNo ratings yet
- Math MeasurementDocument5 pagesMath Measurementapi-444635950No ratings yet
- Handbook of X-Ray SpectrometryDocument985 pagesHandbook of X-Ray Spectrometryjorgehrdz269100% (6)
- Numerical Modeling of The Electrostatic Field in Metal-Insulator-Metal StructuresDocument6 pagesNumerical Modeling of The Electrostatic Field in Metal-Insulator-Metal StructuresEmilia SimonaNo ratings yet
- Aspen Exchanger Design and Rating Shell & Tube V8.8Document3 pagesAspen Exchanger Design and Rating Shell & Tube V8.8Camila Florencia ScarlatoNo ratings yet
- Helmholtz EquationDocument9 pagesHelmholtz EquationAbdul Naser Rafi'i AttamimiNo ratings yet
- Lecture # 1 (PC-1) PDFDocument20 pagesLecture # 1 (PC-1) PDFShreyanshJainNo ratings yet
- Experimental Aero NotesDocument62 pagesExperimental Aero Notesparveensaini100% (1)
- PLP Ps q3 Wk1 Day1Document4 pagesPLP Ps q3 Wk1 Day1Dhulz IlegnaNo ratings yet