Curtin-Hammett Principle: Example
Uploaded by
Dhriti VisvanathanCurtin-Hammett Principle: Example
Uploaded by
Dhriti VisvanathanCurtinHammett principle
From Wikipedia, the free encyclopedia
The CurtinHammett principle is a principle in chemical kinetics that was proposed by David Yarrow Curtin and ouis !lack Hammett" #t states that, for a reaction that has a pair of reactive intermediates or reactants that interconvert rapidly $as is usually the case for conformers%, each &oin& irreversibly to a different product, the product ratio will depend only on the difference in the free ener&y of the transition state &oin& to each product, and not on the e'uilibrium constant between the intermediates"
()*(+*
(edit*,-ample For e-ample, &iven species A and B that e'uilibrate rapidly while A turns irreversibly into C, and B turns irreversibly into D.
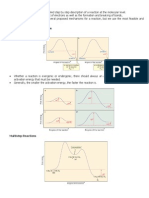
K is the e'uilibrium constant between A and B, and k) and k+ are the rate constants for the formation of C and D, respectively" When the rate of interconversion between / and 0 is much faster than either k) or k+ then the CurtinHammett principle tells us that the C.D product ratio will not reflect K, but the relative ener&y of the transition states" The reaction coordinate free ener&y profile can be represented by the followin& scheme.
The ratio of products will depend only on the value labeled 11 G2 in the fi&ure. C will be the ma3or product, because the ener&y of TS1 is lower than the ener&y of TS2" #t doesn4t matter whether A is more stable than B or not, or by how much" This can be understood 'ualitatively by thinkin& what would happen if the free ener&y of A were increased, while keepin& everythin& else constant" 5n one hand, 1G)2 would become smaller, which would make k) lar&er, therefore favorin& the formation of C" 0ut on the other hand, the amount of A in e'uilibrium would decrease, because the chan&e in 1G would increase the value of K, favorin& B" These two effects cancel out, leadin& to the conclusion that the relative ener&ies of A and B don4t matter" This can also be proved al&ebraically as shown below" (edit*Derivation The rate of formation for compound C from / is &iven as
and that of D from 0 as.
with 6c the e'uilibrium constant" The ratio of the rates is then.
The product ratio can also be written as.
edit*/pplication
to stereoselective reactions
The CurtinHammett principle is used to e-plain the selectivity ratios for stereoselective reactions, such as in kinetic resolution" / typical e-ample is the followin&. a prochiral molecule binds to a chiral catalyst, formin& a pair of diastereomeric intermediates, dependin& on which face of the substrate was bound to the catalyst" These intermediates e'uilibrate rapidly $like A and B in the dia&ram above%, and each one then leads to a different enantiomer of the product throu&h the rate7determinin& step"
1.
^ Carey, Francis /"8 9undber&, :ichard ;"8 $)<=>%" Advanced Organic Chemistry Part A Structure and Mechanisms (2nd ed.). ?ew York ?"Y". !lenum !ress" #90? @7A@B7>))<=7<
You might also like
- NMR Kinetics: Study of A Reversible Hydrolysis Reaction100% (2)NMR Kinetics: Study of A Reversible Hydrolysis Reaction8 pages
- Chapter 2 Kinetics of Homogeneous Reaction EDITEDNo ratings yetChapter 2 Kinetics of Homogeneous Reaction EDITED19 pages
- Bioelectrochemistry: Name: Pranay A Shinde STD: MSC Part 1 ROLL NO: 120 Sub Teacher: Harshada Mam (Physical Chem)No ratings yetBioelectrochemistry: Name: Pranay A Shinde STD: MSC Part 1 ROLL NO: 120 Sub Teacher: Harshada Mam (Physical Chem)17 pages
- Electrocyclic Reactions: Dr. Harish ChopraNo ratings yetElectrocyclic Reactions: Dr. Harish Chopra35 pages
- Irreversible Thermodynamics For Membrane TransportNo ratings yetIrreversible Thermodynamics For Membrane Transport9 pages
- Du Preez BJ - 18387918 - Report - Experiment L3 - The Fries Rearrangement of Phenyl Acetate - Preparing and Isolating Hydroxyphenylacetophenones100% (2)Du Preez BJ - 18387918 - Report - Experiment L3 - The Fries Rearrangement of Phenyl Acetate - Preparing and Isolating Hydroxyphenylacetophenones7 pages
- Thermodynamics of Electrified Interface EquationsNo ratings yetThermodynamics of Electrified Interface Equations15 pages
- Free Radical Substitution and Electrophilic AdditionNo ratings yetFree Radical Substitution and Electrophilic Addition17 pages
- CHEM 334 Quantitative Analysis Laboratory: The Methods of Calibration Curve and Standard AdditionNo ratings yetCHEM 334 Quantitative Analysis Laboratory: The Methods of Calibration Curve and Standard Addition5 pages
- Experiment 4: THE Determination of Partial Molar Enthalpy100% (7)Experiment 4: THE Determination of Partial Molar Enthalpy18 pages
- CHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)No ratings yetCHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)10 pages
- Elimination Reactions Mechanism Lecture NotesNo ratings yetElimination Reactions Mechanism Lecture Notes17 pages
- LCAO MO Theory Illustrated by Its Application To H2No ratings yetLCAO MO Theory Illustrated by Its Application To H28 pages
- Full Download General, Organic, & Biological Chemistry 5th Edition Janice Gorzynski Smith PDF DOCX100% (3)Full Download General, Organic, & Biological Chemistry 5th Edition Janice Gorzynski Smith PDF DOCX57 pages
- An Introduction To Electroanalytical ChemistryNo ratings yetAn Introduction To Electroanalytical Chemistry27 pages
- Journal of Chemical Education Volume 89 Issue 6 2012 [Doi 10.1021_ed200055t] Burgess, Arthur E.; Davidson, John C. -- A Kinetic–Equilibrium Study of a Triiodide Concentration Maximum Formed by the Persulfate–Iodide RNo ratings yetJournal of Chemical Education Volume 89 Issue 6 2012 [Doi 10.1021_ed200055t] Burgess, Arthur E.; Davidson, John C. -- A Kinetic–Equilibrium Study of a Triiodide Concentration Maximum Formed by the Persulfate–Iodide R3 pages
- 03 Werner's Theory of Coordination CompoundsNo ratings yet03 Werner's Theory of Coordination Compounds4 pages
- Chemical Kinetics Reaction Mechanism Frederick LindemannNo ratings yetChemical Kinetics Reaction Mechanism Frederick Lindemann10 pages
- Experiment 2: Determination of A Mixture of Xylene Isomers Using Infrared (Ir) SpectrometerNo ratings yetExperiment 2: Determination of A Mixture of Xylene Isomers Using Infrared (Ir) Spectrometer8 pages
- Week 2. Errors in Chemical Analysis (Abstract)No ratings yetWeek 2. Errors in Chemical Analysis (Abstract)31 pages
- Equations of Change For Non Isothermal SystemsNo ratings yetEquations of Change For Non Isothermal Systems35 pages
- ATOOCV1 3 0 Reaction Mechanism Structure and ReactivityNo ratings yetATOOCV1 3 0 Reaction Mechanism Structure and Reactivity38 pages
- Multidimensional Steric Parameters in The Analysis of Asymmetric Catalytic ReactionsNo ratings yetMultidimensional Steric Parameters in The Analysis of Asymmetric Catalytic Reactions9 pages