0 ratings0% found this document useful (0 votes)
279 viewsRef 111
Ref 111
Uploaded by
AbidHussainBhattiThis document provides an outline for the course "Petroleum Refining Engineering-II" which covers various refinery processes and operations. It discusses the overall refinery scheme including separation processes like distillation, solvent extraction, and treating. Conversion processes such as catalytic cracking, reforming, and coking are also outlined. Key evaluation criteria include exams and assignments. Relevant textbooks and some typical tests on petroleum fractions like octane number, viscosity, and flash point are mentioned as well.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Ref 111
Ref 111
Uploaded by
AbidHussainBhatti0 ratings0% found this document useful (0 votes)
279 views111 pagesThis document provides an outline for the course "Petroleum Refining Engineering-II" which covers various refinery processes and operations. It discusses the overall refinery scheme including separation processes like distillation, solvent extraction, and treating. Conversion processes such as catalytic cracking, reforming, and coking are also outlined. Key evaluation criteria include exams and assignments. Relevant textbooks and some typical tests on petroleum fractions like octane number, viscosity, and flash point are mentioned as well.
Original Description:
Petroleum Refinery Engineering Slides
Original Title
ref 111
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
This document provides an outline for the course "Petroleum Refining Engineering-II" which covers various refinery processes and operations. It discusses the overall refinery scheme including separation processes like distillation, solvent extraction, and treating. Conversion processes such as catalytic cracking, reforming, and coking are also outlined. Key evaluation criteria include exams and assignments. Relevant textbooks and some typical tests on petroleum fractions like octane number, viscosity, and flash point are mentioned as well.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
279 views111 pagesRef 111
Ref 111
Uploaded by
AbidHussainBhattiThis document provides an outline for the course "Petroleum Refining Engineering-II" which covers various refinery processes and operations. It discusses the overall refinery scheme including separation processes like distillation, solvent extraction, and treating. Conversion processes such as catalytic cracking, reforming, and coking are also outlined. Key evaluation criteria include exams and assignments. Relevant textbooks and some typical tests on petroleum fractions like octane number, viscosity, and flash point are mentioned as well.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 111
Petroleum Refining Engineering-II
(CHE425PG 2 credit hours)
2014-Mid-term
Dr. Muhammad Rashid Usman
Institute of Chemical Engineering and Technology
University of the Punjab, Lahore.
Crude oil refining
Iamge taken from: http://heatexchanger-design.com/2011/10/06/heat-exchangers-6/ Dated: 17-Jan-2012
3
Course outline
1. Simplified overall crude oil refinery picture
2. Major refinery products and tests: Brief description
3. Separation process: Atmospheric and vacuum
distillations, lube oil extraction, dewaxing, deasphalting,
and clay treatment.
4. Catalysts used in refinery operations
5. Conversion processes: Brief description of alkylation,
polymerization, isomerization of light paraffins,
hydrotreating, catalytic reforming, catalytic cracking,
hydrocracking, visbreaking of resids, and coking.
5. Material and energy balances for refinery processes:
Simulation of refinery processes
6. Design guidelines for the selected refinery equipment
4
Evaluation
Mid term exam: 35 Marks
Final term exam: 40 Marks
Assignment: 25 Marks
May be one mid-mid term and one mid-final term
exams bearing no marks. The assignment may include
attendance marks, theoretical or experimental problems,
quizzes, etc.
Communication with the instructor will be through
email only. Please see your emails regularly.
Instructor email: mrusman.icet@pu.edu.pk
5
Text books
Gary, J.H.; Handwerk, G.E. 2001. Petroleum refining: Technology
and economics. 4
th
ed. Marcel Dekker, Inc.
Fahim, M.A.; Al-Sahhaf, T.A.; Elkilani, A. 2010. Fundamentals of
petroleum refining. Elsevier.
6
Suggested books
[1] Gary, J.H.; Handwerk, G.E. 2001. Petroleum refining:
Technology and economics. 4
th
ed. Marcel Dekker, Inc.
[2] Fahim, M.A., AlSahhaf, T.A. and Elkilani, A. 2010.
Fundamentals of petroleum refining. Elsevier.
[3] Parkash, S. 2003. Refining processes handbook. Gulf
professional publishing, Elsevier. Singapore.
[4] Wauquier, J.-P. (ed.). 1998. Petroleum refining: Separation
processes. Vol. 2. Technip.
[5] Meyers, R.A. 2004. Handbook of petroleum refining
processes. 3
rd
ed. McGraw-Hill.
7
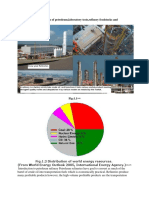
Overall refinery flow [2]
8
Crude oil distillation: Atmospheric
9
Crude oil distillation: Vacuum
Approximate ranges of crude
distillation products [2]
Conversion and separation processes
Separation processes:
Crude distillation (atmospheric distillation and vacuum
distillation), solvent extraction, solvent deasphalting,
solvent dewaxing, and clay treatment.
Conversion processes:
Catalytic reforming, hydrotreating, hydrocracking,
catalytic cracking, alkylation, isomerization, delayed
coking, flexicoking, and visbreaking.
Refinery processes
12
Separation processes
Atmospheric distillation: Desalted crude oil is flashed in the
atmospheric distillation unit and the crude oil is fractionated into
various fractions. Light gases, light and heavy naphthas,
kerosene, light and heavy gas oils, and atmospheric residuum
may be the principal fractions.
Vacuum distillation: The atmospheric residue is vacuum
fractionated and vacuum gas oil and vacuum residuum may be
the products.
Solvent deasphalting: A solvent usually liquid propane is
employed to remove asphaltenes from heavy crude fractions such
as vacuum resid.
Solvent dewaxing: Paraffins of high molecular weight (wax) are
removed from the lube oil stock to adjust the pour point.
13
Separation processes
Solvent extraction: Lube oil stock is treated with a solvent and
aromatics are removed.
Clay treatment: Lube oil stocks are subjected to clay treatment
to remove impurities to better stabilize and to improve the color.
14
Conversion processes
Hydrotreating: It is carried out to remove impurities such as
sulfur, nitrogen, halides etc.
Isomerization: For example, n-butane is isomerized to isobutane
to feed the alkylation plant. n-hexane is isomerized to branched
alkanes to produce a high octane rating product.
Catalytic reforming: It is used to process low grade (octane
number) fraction such as straight run gasoline and naphthas to
produce high grade gasoline range products. Dehydrogenation,
isomerization, and hydrocracking may occur during the course of
catalytic reforming.
Alkylation: As an example, it is the addition of isobutane to
butenes to produce high grade gasoline range product (alkylate).
15
Conversion processes
Catalytic cracking: The catalytic cracking of heavy oil fractions
to produce mainly of gasoline range products.
Hydrocracking: The cracking of heavy oil fractions to produce
low boiling products in the presence of hydrogen and catalyst.
Coking: It is severe thermal cracking that results in light gases,,
coker naphtha, and solid coke.
Visbreaking: It stands for viscosity breaking. Liquid phase mild
thermal cracking of heavy feedstocks.
16
Conversion and separation processes
The crude oil is heated in a furnace and flash-charged to an
atmospheric distillation tower (ADU). Here, it is separated into
light wet gases, unstabilized light naphtha, heavy naphtha,
kerosene, light and heavy atmospheric gas oils, and atmospheric
reduced crude.
The atmospheric reduced crude enters the vacuum reduced
distillation column (VDU) and separated into vacuum gas oil
streams and vacuum reduced crude.
The vacuum reduced crude is sent to a coker where it is
thermally cracked to produce wet gas, gasoline and gas oil range
products and solid coke.
The gas oil ranged products from the ADU and VDU and gas oil
from the coking process are subjected to catalytic and
hydrocracking. The purpose is usually to produce products of
gasoline and diesel range.
17
Conversion and separation processes
The light naphtha streams from the crude tower, coker and
cracking units are sent to an isomerization unit to convert
straight-chain paraffins to isomers that have higher octane
numbers [1].
The heavy naphtha streams from the crude tower, coker and
cracking units are fed to the catalytic reformer to improve their
octane numbers [1].
The wet gases streams from the crude units, coker, and
cracking units are separated in the vapor recovery section (gas
plant) into fuel gas, liquefied petroleum gas (LPG), unsaturated
hydrocarbons (propylene, butylenes, and pentenes), normal
butane, and isobutane. The fuel gas is burned as a fuel in refinery
furnaces and normal butane is blended into gasoline or LPG. The
unsaturated hydrocarbons and isobutane are sent to the alkylation
unit for processing [1].
18
Conversion and separation processes
In some refineries, the heavy vacuum gas oil and reduced
crude from paraffinic or naphthenic base crude oils are processed
into lubricating oils [1].
The vacuum gas oils and deasphalted stocks are first solvent-
extracted to remove aromatic compounds and then dewaxed to
improve the pour point. They are then treated with special clays
or high-severity hydrotreating to improve their color and stability
before being blended into lubricating oils [1].
19
Optimum refinery operation
Each refinery has its own unique processing scheme which is
determined by the process equipment available, crude oil
characteristics, operating costs, and product demand. The
optimum flow pattern for any refinery is dictated by economic
considerations and no two refineries are identical in their
operation. [1]
20
Some crude oils of Pakistan
(From thesis of my student Ahmad)
21
Refining facility in Pakistan
Byco 35,000 bbl/day
ARL 40,000 bbl/day
PRL 50,000 bbl/day
NRL 65,000 bbl/day
PARCO 100,000 bbl/day
Byco has added nearly 120,000 bbl/day capacity in its new
installation.
In the past, further new installations were expected including KCR,
Indus, and Trans Asia.
22
General refinery products
Refinery fuel gas
Liquefied petroleum gas
Solvent naphtha
Gasoline
Kerosene
Jet fuel or gas turbine fuel
Diesel fuel
Fuel oil
Residual fuel oil
Lubricating oil or lube oil
Wax
Asphalt
Petroleum coke
23
Refinery products: Brief description[2]
24
Tests on petroleum fraction
Pour point: A lower pour point means paraffinic content is low.
It is a measure of ease or difficulty of a fraction to be pumped in
cold conditions.
Viscosity: It is usually measured in centi Stokes or Saybolt
seconds at 37.8 and 99 C. These two points are used to find
viscosity index of the fraction.
Aniline point: It is an indication of the amount of the aromatic
content in a given fraction.
Flash point: It is important for gasoline and naphtha.
Octane number: Motor octane number (MON) is the test carried
out at high speed (900 rpm). Research octane number (RON) is
measured at low speed (600 rpm). PON (posted octane number is
the arithmetic average of RON and MON).
25
Tests on petroleum fraction
Reid vapor pressure: Vapor pressure determined in a volume of
air four times the liquid volume at 37.8 C (100 F). It indicates
vapor lock characteristics and explosion hazards.
Carbon residue:
Smoke point: It is a measure of the burning qualities of kerosene
and jet fuels. It is measured in terms of the maximum height in
mm of a smokeless flame of fuel.
Refractive index: It is the ratio of the velocity of light in vacuum
to the velocity of light in the oil. It is a used to characterize a
petroleum fraction.
Cetane number: It measures the ability for autoingnition in
diesel (compression ignition) engines. It is the percentage of pure
cetane (n-hexadecane) in a blend of cetane and alpha methyl
naphthalene which corresponds to the ignition characterstics of a
given diesel sample.
26
Tests on petroleum fraction
Freezing point: It is the temperature at which the hydrocarbon
liquid solidifies at atmospheric pressure. It is one of the important
property specifications for kerosene and jet fuels due to the low
temperatures encountered at high altitudes in jet planes.
Sediments: These are solid materials that are not soluble in the
hydrocarbon or water and can be comprised of sand, drilling
mud, rock or minerals, particles from erosion of metal pipes,
tanks, and other process equipments.
27
Separation processes
Generally, separation processes may be classified as
either mechanical-physical separation processes or mass
transfer operations.
o Mechanical-physical separation processes
(do not require a mass transfer gradient for the
separation)
o Mass transfer operations
(based on diffusion and require a mass transfer
gradient for the separation)
28
Separation processes
Examples of mechanical-physical separation processes
are:
Size reduction
Size enlargement (not crystallization)
Size separation (screening, etc.)
Filtration
Some and not all membrane separation processes
Sedimentation (Thickening and clarification)
Elutriation
Floatation
Centrifugation
29
Separation processes
Examples of mass transfer operations are:
Distillation
Drying
Liquid-liquid extraction
Leaching or lixiviation
Gas absorption
Membrane separation
(Not all membrane separation processes)
Humidification
Adsorption
30
Atmospheric distillation unit
(ADU)
What is distillation?
Why do we need distillation of
a crude oil?
31
Atmospheric distillation unit (ADU)[3]
32
Atmospheric distillation unit (ADU)
The column operates generally at a pressure greater than
atmospheric pressure. This may be done for pressure drop
considerations, to flow the vapors from one location to the
desired location, and/or cooling water to be used for the
overhead condenser. A higher pressure increases the
bubble point.
What is the criteria for setting pressure in the
overhead condenser (column)?
33
Atmospheric distillation unit (ADU)
Nearly 5060% of the crude oil is vaporized in the flash
zone of the tower [3].
A preflash tower is sometimes added before the
atmospheric column, if the crude oil contains appreciable
amounts of lighter products.
How is this advantageous?
The bottom temperature is bounded in the range of 700-
750 F [3]. This is done avoid cracking.
The superheated steam required to boil off the crude
bottoms is usually at about 600 F [3].
The steam consumption is usually 510 lb/bbl of
stripped product [3].
34
Vacuum distillation unit (VDU)
Overflash: 5 to 10% of the bottoms that acts as an internal
reflux to better fractionate the few trays above the flashzone.
Pumparound reflux is used to remove heat from the
column. A stream at a higher temperature in a column is
taken out of the column, exchanges heat with the crude oil
feed and heats it, and then returns back to the column at
some higher position in the column (lower temperature).
35
Atmospheric distillation unit
(ADU)
Most atmospheric towers contain 2535 trays between the
flash zone and the top tower.
The allowable pressure drop for trays is approximately
0.10.2 psi per tray. Generally a pressure drop of 5 psi is
allowed between the flash zone and the top tower.
Ref.: 3
36
Atmospheric distillation unit (ADU)[3]
37
Vacuum distillation unit (VDU)[1]
38
Vacuum distillation unit (VDU)
The temperature required at the furnace outlet for
atmospheric distillation unit (ADU) will be excessive if
the heavier fractions will be distilled in the ADU. This
will be resulted in thermal cracking and loss of the
product and fouling of the equipment. This necessitates
the use of vacuum distillation column where the
distillation occurs under sub-atmospheric conditions.
Decreasing pressure of a component decreases the
boiling point and vice versa.
39
Vacuum distillation unit (VDU)
Furnace outlet temperatures are usually in range of 730
850 F (388454 C) [1].
The pressure in the flash zone is around 2540 mm Hg [1].
The effective pressure for hydrocarbon vaporization is
reduced by using stripping steam in the furnace as well as at
the tower bottom. The steam added to the furnace increases
the velocity of the fluid and decreasing the coke formation.
The steam consumption is usually 1050 lb/bbl of feed [1].
The lower pressure in the tower increases the diameter of
column. A higher pressure increases the boiling
temperatures and difficulty of separation. Vacuum
distillation columns have large diameters and the diameter
may easily reach 40 ft [1].
40
Vacuum distillation unit (VDU)[3]
41
Vacuum distillation unit (VDU)[3]
42
Vacuum distillation unit (VDU)
The gases produced at the top of the column are sent to the
fired heater and burned to release heat.
The gases separated from the sour water in foul water
stripper are sent to the flare system. Part of the water
recovered in the stripper is sent to the desalters.
Vacuum gas oil produced may be sent to hydrocracking,
catalytic cracking, or lube oil processing.
What for vacuum residue?
43
Vacuum distillation unit (VDU)
It is essential to design the fractionator tower, overhead
lines, and condenser to minimize the pressure drop between
the vacuum-inducing device and the flashzone. A few
millimeters decrease in pressure drop will save many
dollars [1]. Structured packings may be employed in the
column as having high HETP and offer low pressure drop.
The desired operating pressure in column is maintained
using steam ejectors and barometric condensers or vacuum
pimps and surface condensers. For a flash zone pressure of
25 mmHg, three ejector stages are usually required [1].
The vacuum produced is limited to the vapor pressure of
the water used in the condensers[1].
44
Vacuum distillation unit (VDU): Examples of structured packings [7]
45
Vacuum distillation unit (VDU):
Steam ejector
46
Vacuum distillation unit (VDU): Barometric condenser
47
Separation processes
(Lube oil processing)
What is a lubricating (lube) oil?
What is its function?
48
Separation processes
(Lube oil processing)
What should be the important properties
of lube oils?
1. Viscosity
2. Viscosity change with temperature (viscosity index)
3. Pour point
4. Oxidation resistance
5. Flash point
6. Boiling temperature
7. Acidity (neutralization number)
8. Thermal stability
8. Color
See Chapter 14 of Ref. 1.
49
Separation processes
(Lube oil processing)
Viscosity: Higher the viscosity, the thicker the film of the
oil. However, too high a viscosity may cause undesirable
friction and heat. Higher the boiling range of the fraction,
usually greater the viscosity.
What boiling range should be selected for a lube oil that
requires a high viscosity?
Viscosity index: Higher the viscosity index, the smaller the
change in viscosity with temperature. It may a negative or
even greater than 100. Additives such as polyisobutylenes
and polymethacrylic acid esters are added to improve the
vscosity index characteristics.
50
Separation processes
(Lube oil processing)
Pour point: For motor oils, a low pour point is very
important to obtain ease of starting and proper start up
lubrication on cold days.
Oxidation resistance: At high temperatures such as in an
internal combustion engine can cause rapid oxidation.
Oxidation causes the coke formation and the formation of
asphaltic type materials. These procuts can injure the metal
surfaces and can block the flow lines. Additives such as
phenolic compounds and zinc dithiophosphates are added to
inhibit the oxidation reactions.
51
Separation processes
(Lube oil processing)
Flash point: It may indicate the type of hydrocarbons are
present in the lube oil and determines the volatility of the oil
and the possible emissions it may cause.
Boiling point: It tells the type of the hydrocarbons present
and gives an idea of the viscosity of the oil.
Acidity: Organic acids formed by the oxidation of oil or that
formed as byproducts in the combustion of oil may cause corrosion
and therefore needed to be reduced. Lube oil blending stocks from
paraffinic crude oils have excellent thermal and oxidation stability
and exhibit lower acidity than do the oils from the naphthenic crude
oils. Acidity characteristics are determined by neutralization
number. Can you define acid number?
52
Separation processes
(Lube oil processing)
Thermal stability: It ensures that the lubricating oil is
stable under the conditions of operation and not cracked or
reactive.
Color: Reasonably unacceptable color may a sign of the
presence of olefins, metal complexes, and heteroatoms such
as nitrogen
53
Separation processes
(Lube oil processing)
Undesirable characteristics of raw lube oils [1]:
1. High cloud and pour points
2. Large viscosity change with temperature, i.e., low
viscosity index
3. Poor oxidation resistance
4. Poor color
6. High organic acidity
7. High coke forming and sludge forming ability
54
Definitions of lube oils
Neutral oil:
Straight run distillation fraction suitable for lube oil
products.
Bright stock:
Deasphlated vacuum residue suitable for lube oil.
Finished lube oil:
The blended lube oil product of final desired properties.
We will go through here separation processes only.
Paraffinic lube oils: These are all grades of lube oils, from
both neutral and bright stocks, that have a finished viscosity
index greater than 75. [6]
Naphthenic lube oils: Lube oils with a finished viscosity
index of less than 75 [6].
55
Separation processes
(Lube oil processing)
The processes used to change the above mentioned
undesirable properties of the raw lube oils are as follows:
1. Solvent deasphalting
2. Solvent extraction or Hydrocracking
3. Solvent dewaxing or selective hydrocracking
(hydrodewaxing)
4. Clay treatment or Hydrotreating
The modern trend is to use hydroprocesses, however, we will go
through separation processes here and similar hydroprocesses will
be discussed in rather detail in the final term.
56
Separation processes
(Lube oil processing)
57
Atomic H/C ratios of various species [8]
58
Solvent deasphalting
The purpose of deasphalting is to remove asphaltenes and resins
and that it is required to reduce metallic contents and coke and
sludge formation tendencies. For maximizing fuel (in fuel
refinery) or when the vacuum fraction is not suitable for lube oil
production, the deasphalting is carried out to prepare feed for the
subsequent conversion processes. So, it is not always carried out
for preparing lube oil stock. Owing to the high carbon residues
and metal contents (sulfur contents may well be) of the
asphaltene and resin fraction, removal of asphaltenes and resins
reduces these contents in the feed for catalytic conversion units.
Catalysts involved in the conversion processes may be damaged
and greatly deactivated in the presence of metals and high carbon
residue. Severe fouling of the process equipment may also be a
reason.
59
Solvent deasphalting
Removal of sulfur, metal, and nitrogen from a vacuum
residue is usually less expansive by deasphalting than by
hydrotreating. The desulfurized, demetallized, etc. residue
may be used as a blending stock for the feed for
hydrocracking and catalytic cracking.
The light lube oil fractions, if any, can avoid deasphalting and
directly treated in solvent extraction.
60
Solvent deasphalting
From: http://www.pcs.gr.jp/doc/EMousse/text.htm
61
Asphaltenes
(Solvent deasphalting)
From: The shell bitumen industrial handbook, p. 53.
62
Resins
(Solvent deasphalting)
From: The shell bitumen industrial handbook, p. 53.
63
Propane deasphalting [1]
64
Rotating disc contactor (RDC)
From: http://www.liquid-extraction.com/rdc-column.htm
65
Rotating disc contactor (RDC) [5]
66
Propane deasphalting [1]
o Feed is mixed with a small amount of solvent to increase
the fluidity of the feed.
o The feedstock is usually treated with 4 to 8 volumes of
liquid propane.
o The extractor is usually a baffle column or rotating disc
contactor.
Name the other types of column extractors.
o The extract phase contains from 15 to 20% by weight of
oil with the remainder solvent. The raffinate phase
contains from 30 to 50% propane by volume.
o The heavier the feedstock, the higher the ratio of
propane to oil required.
67
Propane deasphalting
o The propane deasphalting tower is operated at a
pressure sufficiently high to maintain the solvent in the
liquid phase. This is usually about 500 psig (3448 kPa).
[1].
o The critical temperature of propane is 96.8 C so the
upper limit may have to be set below this temperature
(gases cannot be liquefied above critical temperature).
The temperature is usually limited to 82 C [8].
o Solvent to oil ratio is a function of feedstock properties
and the required product specifications. An increase in
the solvent to oil ratio increases the product quality.
68
Selection of solvent for liquid-liquid
extraction: Class input
1. Start
69
Selection of solvent for liquid-liquid
extraction
1. High solubility of the solute in the solvent but low
solubility or preferably immiscibility with feed solvent
2. Should not be reactive with the feed
3. Phases should have high density difference.
4. Solute and solvent should be economically separable.
5. High distribution coefficients
6. Should be stable and non-volatile under the conditions of
operation
7. Should have low cost
8. Easy and regular availability (no inventory problems)
9. Non-corrosive, non-toxic, and environmental friendly.
70
Selection of solvent for liquid-liquid
extraction
Do you have an idea of ternary phase
diagrams used in liquid-liquid extraction?
71
Propane deasphalting
How does the solvent to feed ratio affect the
design and performance of a liquid-liquid
extractor?
What are dispersed phase, continuous phase,
flooding, dispersed phase holdup, mass transfer
efficiency (overall mass transfer coefficient or
overall height of transfer unit), interfacial tension,
drop diameter, and drop distribution in relation to a
continuous liquid-liquid extractor?
72
Propane deasphalting
Propane, usually, is used as the solvent in deasphalting but
it may also be used with ethane or butane in order to obtain
the desired solvent properties. Propane has unusual solvent
properties in that from 100 to 140 F (40 to 60 C) paraffins
are very soluble in propane, but the solubility decreases with
an increase temperature until at the critical temperature of
propane [206 F (96.8 C)] all hydrocarbons become
insoluble. In the range of 100 to 206 F (40 to 96.8 C) the
high molecular weight asphaltenes and resins are largely
insoluble in propane. [1]
73
Propane deasphalting
As the metal, sulfur, and nitrogen are generally concentrated
in the larger molecules, the metal, sulfur, and nitrogen
content of deasphalted oil is considerably reduced as shown
in the next slide [3].
Asphalt may be burned to produce energy, but due to
fluidity problems and stack gas issues (high cost for gas
cleaning) it is commonly used for road paving, water
proofing, and insulation. Asphalt may be air treated (asphalt
blowing) to improve its properties.
74
Propane deasphalting [3]
75
Propane deasphalting [8]
76
Propane deasphalting [3]
77
Characteristics of various families
in lube oil stock [4]
78
Characteristics of various families in
lube oil stock [1]
79
Solvent extraction: Purposes
(lube oil extraction) [1]
The purpose of solvent extraction is to remove the aromatics
from the lube oil fraction and in doing so to improve the:
1. Viscosity index (VI)
2. Oxidation resistance
3. Color
4. Coke forming tendency, and
5. Sludge forming tendency.
80
Solvents commonly applied
1. Furfural
2. Phenol
3. N-methyl-2-pyrrolidone (NMP)
81
Comparison between the three
solvents [4]
82
Phenol extraction [1]
Phenol being toxic is not recommend.
83
Comparison between the NMP and
furfural
NMP
Furfural
84
NMP advantages over furfural [4]
Better stability
Better oxidation resistance
Less carryover in the raffinate and the
extract
Higher solvent power towards aromatics
Lower process temperature
Less toxicity
85
NMP advantages over furfural [4]
o Since NMP is much more stable than furfural, the
pretreatment (deaeration) section is unnecessary and the
feed can directly be let into the extraction tower.
o Solvent ratios in an NMP unit are significantly lower
than for furfural, i.e., it has higher solvent power. A
smaller plant size is therefore required.
o Solvent injection temperatures are lower by 10 to 20 C
for the same final viscosity index (VI) and an identical
raffinate yield.
o Furfural is sensitive to oxidation and to the presence of
water, which significantly lower extraction performance.
86
NMP disadvantages over furfural [4]
Lower specific gravity
Less selectivity
Higher boiling points
NMPs higher boiling point and heat of
vaporization requires higher temperatures
and energy consumption is also greater than
for furfural recovery.
87
Simplified furfural extraction process [6]
88
Rather detailed furfural extraction process [9]
89
Furfural extraction
The furfural is fed at the top while the oil flows
countercurrently from the bottom.
The extractor is commonly a packed column with
Raschig rings or an RDC [1].
The oil behaves as a continuous phase while furfural
acts as a dispersed phase.
Furfural to oil ratio ranges between 2:1 for light stocks
and 4.5:1 for heavy stocks [1].
90
Factors for furfural extraction
process
Furfural to oil ratio
Extraction temperature depends upon the feed
characteristics and adjust the viscosity of the oil-furfural
mixture and miscibility of the furfural with oil.
A part of the extract phase may be recycled and may
affect the efficiency of the extraction.
The extraction column operates between 50 and 200
psig [6] and at a top temperature of 105 to 150 C [1].
The temperature gradient betweeen top and bottom of
the extractor is between 30 to 50 C [1].
91
Furfural extraction [6]
92
NMP extraction [4]
93
NMP extraction [4]
94
NMP extraction: Water addition [4]
NMP solvent power is very high towards aromatics, and
also considerable towards paraffins, which consequently
lowers the raffinate yield. To attenuate the solvent power
of NMP, a small amount of water (0.8 to 3.0 wt%) is
added and the solvent that circulates in the unit is a
mixture of NMP and water. The amount of water required
in the solvent depends on the target level of the extraction.
95
Factors for furfural extraction
process
As mentioned before phenol is toxic and no more
required for the extraction process in the existing
or new installations.
Give your suggestions to modify an existing
phenol extraction plant to be replaced by an
NMP extraction plant.
96
Dewaxing [1, 4]
The straight chain and slightly branched paraffinic
compounds tend to crystallize under ordinary
temperature conditions. However, at low
temperature such as 20 C, the oil needs to be in a
liquid state in the engine crankcase. If crystallize
or solidify, it will cause flow problems.
97
Dewaxing [1,4]
The dewaxing unit is required to lower the
cloud and pour points of the oil by
eliminating the compounds that crystallize at
relatively higher temperature.
98
Solvent dewaxing
The solvent reduces the viscosity of the oil and
facilitate in pumping and filtration.
The most common dewaxing process is based
on crystallization with a solvent that modifies the
conditions of thermodynamic equilibrium solely
by its presence in the liquid. The ideal solvent
should dissolve the oil and precipitate all the
wax. Additionally, in precipitating the wax
crystal structure should be loose so that oil can be
filtered through the wax. [6]
99
Dewaxing: Solvents [1, 4]
Mixture of methylethylketone (MEK) and
toluene
Methylisobutylketone (MIBK)
Dichloromethane
Trichloroethylene
Propane
Mixture of ethylene chloride and benzene
Mixture of acetone and benzene
100
Dewaxing: Solvents [1, 4]
MEK displays low solvent power for
paraffinic compounds (and therefore good
selectivity)
Toluene has excellent solvent power for
base stocks
The proportions in the mixture of these two
solvents can be optimized.
101
Dewaxing: Solvents [9]
102
Simplified dewaxing PFD [4]
Slack wax
103
Simplified dewaxing process [4]
Contacting the solvent with oil
Crystallization in the presence of the
solvent
Filtration to remove wax from dewaxed oil
Separation of the solvent from the dewaxed
oil and the wax by distillation.
104
Simplified dewaxing process [4]
The feedstock is mixed with the solvent such as
mixture of MEK and toluene.
The mixture is heated to make a mixed of oil and
solvent.
The mixture is then cooled and chilled in heat
exchanger and chiller system. The chiller is usually a
refrigeration system operating at propane.
The mixture of crystals, oil, and solvent flows to the
rotary filters. The cakes are washed with solvent to
purify the wax from oil.
The solvent is recovered in wax and dewaxed oil
distillation columns.
105
Rotary drum vacuum filter [10]
106
Dewaxing Case Study [9]
Feed
107
Clay treatment [1, 4]
Clay treatment is an adsorption process which
is used to remove
Colored compounds
Organic acids
Oxidizable hydrocarbons
Organic nitrogen compounds importantly
affect the color and color stability oil.
108
Clay treatment [1, 4]
Clay treatment may either be carried out by:
Percolation technique such as flow
through a packed particle (adsorbent) bed
Contact process
In the contact process the oil and clay are
mixed, heated, agitated, and filtered.
109
Clay treatment [1, 4]
Spent clay disposal issues may be one of the
major reasons for replacing clay treatment
with an increasingly popular hydrogen
treatment (hydrofinishing).
110
Characteristics of some commercial
grade lube oils [9]
111
References
[1] Gary, J.H. and Handwerk, G.E. 2001. Petroleum refining: Technology and
economics. 4
th
ed. Marcel Dekker, Inc.
[2] Fahim, M.A., AlSahhaf, T.A. and Elkilani, A. 2010. Fundamentals of petroleum
refining. Elsevier.
[3] Parkash, S. 2003. Refining processes handbook. Gulf professional publishing,
Elsevier. Singapore.
[4] Wauquier, J.-P. (ed.). 1998. Petroleum refining: Separation processes. Vol. 2.
Technip.
[5] Myers, R.A. 2004. Handbook of petroleum refining processes. 3
rd
ed. McGraw-
Hill.
[6] Stan Jones, D.S.J. and Pujad, P.R. 2006. Handbook of petroleum processing.
Spinger.
[7] Seader, J.D.; Henley, E.J.; Roper, D.K. 2011. Separation process principles:
Chemical and biochemical operations. 3
rd
ed., John Wiley & Sons, Inc.
[8] Speight, J.G.; Ozum, B. 2002. Petroleum refining processes. Marcel Dekker, Inc.
[9] Parakash, S. 2010. Petroleum Fuels Manufacturing Handbook. McGraw-Hill, Inc.
[10] Brown, G.G. and others. 1950. Unit operations, Wiley & Sons, Inc.
You might also like
- GB3077-1999 English VersionDocument20 pagesGB3077-1999 English VersionHermanto SupuNo ratings yet
- Hydrocracking TechnologyDocument11 pagesHydrocracking TechnologyAsad SaeedNo ratings yet
- PRE 2 MidT SepProc 10 Jun 2014 PArt 1Document34 pagesPRE 2 MidT SepProc 10 Jun 2014 PArt 1Shabbir AliNo ratings yet
- Pre 2 30 July 2016 160731090013 PDFDocument284 pagesPre 2 30 July 2016 160731090013 PDFridanorma100% (1)
- Unit-02 Petroleum Process I-IVDocument143 pagesUnit-02 Petroleum Process I-IVMayank Koparkar100% (1)
- 5 - Refinery Process FlowDocument16 pages5 - Refinery Process Flowimdevil1206No ratings yet
- Overview - PREDocument19 pagesOverview - PREayush gandhiNo ratings yet
- Petroleum IndustryDocument39 pagesPetroleum IndustryQuenie Rose RontalNo ratings yet
- Classification of Crude OilDocument6 pagesClassification of Crude OilSultana AlmansooriNo ratings yet
- DownstreamDocument62 pagesDownstreamKhairul IrfanNo ratings yet
- Petroleum Refining Crude Oil Refining Processes PDFDocument6 pagesPetroleum Refining Crude Oil Refining Processes PDFJAPAN NANAVATI0% (1)
- Crude Oil PlantDocument10 pagesCrude Oil PlantEkbal 6MNo ratings yet
- Oil Refinery ProcessDocument13 pagesOil Refinery Processmch4561696% (26)
- OperationDocument11 pagesOperationmahboobiqbal09No ratings yet
- UntitledDocument7 pagesUntitledapi-256504985No ratings yet
- Petroleum: Chemistry and Technology OFDocument83 pagesPetroleum: Chemistry and Technology OFMohamed MoüsaNo ratings yet
- Lecture Notes OEDocument33 pagesLecture Notes OEbot4.me.84No ratings yet
- Petroleum RefiningDocument32 pagesPetroleum RefiningShashank Tewari100% (14)
- Oil RefineryDocument9 pagesOil RefineryArun BashkarNo ratings yet
- Purifier Maintenance 1Document54 pagesPurifier Maintenance 1Noel Nico FernandoNo ratings yet
- Diesel Fuel Refining and Its AdvancementDocument21 pagesDiesel Fuel Refining and Its Advancementvivek20072008No ratings yet
- Chemistry and Technology of PetroleumDocument83 pagesChemistry and Technology of PetroleumManish TiwariNo ratings yet
- Fertilizer PlantDocument22 pagesFertilizer PlantvictorNo ratings yet
- Hydrocarbon Processing Refining Processing 2004Document293 pagesHydrocarbon Processing Refining Processing 2004Anonymous I29NP3c100% (1)
- FALLSEM2020-21 CHE1014 TH VL2020210101682 Reference Material I 19-Aug-2020 Catalytic Cracking Different Types PDFDocument77 pagesFALLSEM2020-21 CHE1014 TH VL2020210101682 Reference Material I 19-Aug-2020 Catalytic Cracking Different Types PDFJateni GedaNo ratings yet
- Catalytic Cracking PDFDocument96 pagesCatalytic Cracking PDFSri NivasNo ratings yet
- Quiz Mohd Rafiq Mohd ZubirDocument11 pagesQuiz Mohd Rafiq Mohd ZubirMohd RafiqNo ratings yet
- Crude Oil Refining UpgradingDocument13 pagesCrude Oil Refining Upgradinglalitpardasani3556No ratings yet
- Petroleum Refining and Economics: Kobbina Awuah (Machinery Engineer/Project Manager, Conocophillips-Bayway Refinery)Document21 pagesPetroleum Refining and Economics: Kobbina Awuah (Machinery Engineer/Project Manager, Conocophillips-Bayway Refinery)GNo ratings yet
- Unit 1 Characterisation of Crude: HE Etroleum EchnologyDocument54 pagesUnit 1 Characterisation of Crude: HE Etroleum EchnologyJateni GedaNo ratings yet
- Introduction To RefineryDocument6 pagesIntroduction To RefinerydyarNo ratings yet
- Key Terms DefinitionDocument6 pagesKey Terms DefinitionHammad HashmiNo ratings yet
- Fluid Catalytic Cracking ProcessDocument3 pagesFluid Catalytic Cracking ProcessMinh Tuấn PhạmNo ratings yet
- Guide To Refinery ProcessDocument131 pagesGuide To Refinery Processvazzoleralex6884100% (2)
- Chemistry AIP - A Overview On RIL RefineryDocument19 pagesChemistry AIP - A Overview On RIL RefinerySamanyuNo ratings yet
- Oil Refining 04Document16 pagesOil Refining 04Patel Ashok100% (1)
- Presentation of Gasoline (Petroleum Refinery)Document17 pagesPresentation of Gasoline (Petroleum Refinery)hayder alaliNo ratings yet
- Refinery BasicsDocument27 pagesRefinery BasicsArsalan QadirNo ratings yet
- 002 Lecture OverView Refinery Lecture B W 002Document87 pages002 Lecture OverView Refinery Lecture B W 002Hassan ShahidNo ratings yet
- Oil Refinery Processes-1Document13 pagesOil Refinery Processes-1Sai KiranNo ratings yet
- Petroleum Chemistry and Its Refineries05Document21 pagesPetroleum Chemistry and Its Refineries05Louis ThianNo ratings yet
- Petroleum Refining Process Control and Real-Time OptimizationDocument11 pagesPetroleum Refining Process Control and Real-Time OptimizationLuís Roberto Cavalcanti da SilvaNo ratings yet
- Liquid Fuels PDFDocument48 pagesLiquid Fuels PDFAABID SHAIKNo ratings yet
- Petrochemical ProcessDocument20 pagesPetrochemical Processsanjeevs01No ratings yet
- Hydrocarbon ProcessingDocument20 pagesHydrocarbon Processingsanjeevs01No ratings yet
- Light, Intermediate, Heavy Distillates, and Residues. Light DistillatesDocument8 pagesLight, Intermediate, Heavy Distillates, and Residues. Light DistillatesRowel GanzonNo ratings yet
- Oil RefineryDocument9 pagesOil RefineryprathapNo ratings yet
- A Guide of Refinery ProcessDocument32 pagesA Guide of Refinery Processnomurapre100% (1)
- Introduction:Composition of Petroleum, Laboratory Tests, Refinery Feedstocks and ProductsDocument17 pagesIntroduction:Composition of Petroleum, Laboratory Tests, Refinery Feedstocks and ProductsZaid YahyaNo ratings yet
- Oil-Degassing:: 1-SeparationDocument34 pagesOil-Degassing:: 1-SeparationAhmed AbdullaNo ratings yet
- PetroluemDocument37 pagesPetroluemAhmed AbdullaNo ratings yet
- Lecture 3 2024Document37 pagesLecture 3 2024karamysovernar2208No ratings yet
- Fundamentals of Refining and Petrochemicals ProcessesDocument263 pagesFundamentals of Refining and Petrochemicals Processesneocentricgenius100% (1)
- CrackingDocument20 pagesCrackingNiaz Ali KhanNo ratings yet
- Ch8 Refinery ProcessesDocument46 pagesCh8 Refinery Processesفرح100% (1)
- Define Steam Reforming Process?: Chemical Reaction Carbon Monoxide Water Vapor Carbon Dioxide HydrogenDocument7 pagesDefine Steam Reforming Process?: Chemical Reaction Carbon Monoxide Water Vapor Carbon Dioxide Hydrogenjeevanantham 5846No ratings yet
- Lecture 1Document22 pagesLecture 1Amit Narayan RaiNo ratings yet
- Arc Welding: Nauman Ahmad SEN-EE Lecturer UMT LahoreDocument11 pagesArc Welding: Nauman Ahmad SEN-EE Lecturer UMT LahoreJamil AhmadNo ratings yet
- Aceite Hidraulico Mobil DTE 26Document2 pagesAceite Hidraulico Mobil DTE 26Carlos Andres Gonzalez GarciaNo ratings yet
- DetailsDocument2 pagesDetailsMecirdi Mohamed el AmineNo ratings yet
- Paper Mill ReportDocument37 pagesPaper Mill Reportdibyakeshari890No ratings yet
- BITLPFS.02 Quality Standard For Efusion Fusion Bonded Epoxy Coating Rev 03Document2 pagesBITLPFS.02 Quality Standard For Efusion Fusion Bonded Epoxy Coating Rev 03proyectostrillo1No ratings yet
- LAUNDRYDocument29 pagesLAUNDRYUtkarsh NagNo ratings yet
- Cenpes: Index of Revisions REV Description And/Or Revised SheetsDocument30 pagesCenpes: Index of Revisions REV Description And/Or Revised SheetsOrlando PWRNo ratings yet
- Dr. Chaitanya Sharma Phd. Iit RoorkeeDocument62 pagesDr. Chaitanya Sharma Phd. Iit RoorkeeTrung Quoc Le100% (1)
- ATAS Color ChartDocument2 pagesATAS Color ChartKirito SeiyaNo ratings yet
- NDT REQUEST Piping Sistem SPU B Phase 1 (Road Crossing)Document3 pagesNDT REQUEST Piping Sistem SPU B Phase 1 (Road Crossing)Ferdie OSNo ratings yet
- Solidification of Castings-FDocument7 pagesSolidification of Castings-FAshok PradhanNo ratings yet
- Welding Carbon Steel Plates Fillet Weld: (Flat, Horizontal Position)Document15 pagesWelding Carbon Steel Plates Fillet Weld: (Flat, Horizontal Position)Vanessa HadJeanxNo ratings yet
- Gas Engines Emissions & Exhaust After-Treatment A&i GuideDocument36 pagesGas Engines Emissions & Exhaust After-Treatment A&i GuidelamNo ratings yet
- Treatment of Effluent From RefineriesDocument26 pagesTreatment of Effluent From RefinerieskalaiNo ratings yet
- Specification For Acmv PipingDocument5 pagesSpecification For Acmv PipingcashloverNo ratings yet
- Internal Combustion EngineDocument35 pagesInternal Combustion EngineMuhammad FaizNo ratings yet
- Stainless Steel StripDocument17 pagesStainless Steel StripMusicNo ratings yet
- Answer Sheet General Chemistry 2 Set 2Document5 pagesAnswer Sheet General Chemistry 2 Set 2Dane HillaryNo ratings yet
- MI L"STD-1595A 26 February 1982 Superseding MIL-STD-1 595 26 July 1977Document72 pagesMI L"STD-1595A 26 February 1982 Superseding MIL-STD-1 595 26 July 1977Jay MillerNo ratings yet
- 01 - Crospovidone As Dry BinderDocument45 pages01 - Crospovidone As Dry BinderPAQUI MIRANDANo ratings yet
- Appendix S - Welding Provisions - BNBC 2020 CommentaryDocument5 pagesAppendix S - Welding Provisions - BNBC 2020 CommentaryTarif Aziz MarufNo ratings yet
- 4 Jaw ChuckDocument3 pages4 Jaw Chuckavksekar_701032284No ratings yet
- Astm A434Document3 pagesAstm A434Evandro Luis Gomes100% (1)
- Ceramic Tile CAD - Detail Document2Document46 pagesCeramic Tile CAD - Detail Document2داروین پرزNo ratings yet
- Analysis of Fabrcation Methods For MGs Based Composites - A ReviewDocument8 pagesAnalysis of Fabrcation Methods For MGs Based Composites - A ReviewVivek ChawlaNo ratings yet
- Sample Heat Treatment ProcedureDocument13 pagesSample Heat Treatment ProcedureAnonymous uXdS9Y7100% (1)
- Ch07 HW QuestionsDocument2 pagesCh07 HW QuestionsSirilak KlakwongNo ratings yet
- Brosur AOTAI 2016Document16 pagesBrosur AOTAI 2016Andres Agung Perdana100% (1)
- Bhai Brochure Cement Flyash Storage Silo - PDF (Semple)Document2 pagesBhai Brochure Cement Flyash Storage Silo - PDF (Semple)Anand PuntambekarNo ratings yet