Elimination Reaction PDF
Elimination Reaction PDF
Uploaded by
shantharmCopyright:
Available Formats
Elimination Reaction PDF
Elimination Reaction PDF
Uploaded by
shantharmOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Elimination Reaction PDF
Elimination Reaction PDF
Uploaded by
shantharmCopyright:
Available Formats
Elimination reaction
The reaction rate, inuenced by both the alkyl halide
and the base (bimolecular), is second order.
Because E2 mechanism results in formation of a
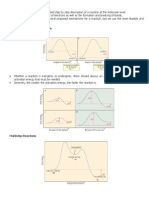
pi bond, the two leaving groups (often a hydrogen and a halogen) need to be antiperiplanar. An
antiperiplanar transition state has staggered conformation with lower energy than a synperiplanar transition state which is in eclipsed conformation with
higher energy. The reaction mechanism involving
staggered conformation is more favorable for E2 reactions (unlike E1 reactions).
Elimination reaction of cyclohexanol to cyclohexene with sulfuric
acid and heat [1]
An elimination reaction is a type of organic reaction
in which two substituents are removed from a molecule
in either a one or two-step mechanism.[2] The one-step
mechanism is known as the E2 reaction, and the two-step
mechanism is known as the E1 reaction. The numbers
do not have to do with the number of steps in the mechanism, but rather the kinetics of the reaction, bimolecular
and unimolecular respectively.
E2 typically uses a strong base, it needs a chemical
strong enough to pull o a weakly acidic hydrogen.
In order for the pi bond to be created, the
hybridization of carbons need to be lowered from
sp3 to sp2 .
In most organic elimination reactions, at least one hydrogen is lost to form the double bond: the unsaturation of
the molecule increases. It is also possible that a molecule
undergoes reductive elimination, by which the valence of
an atom in the molecule decreases by two. An important
class of elimination reactions are those involving alkyl
halides, with good leaving groups, reacting with a Lewis
base to form an alkene. Elimination may be considered
the reverse of an addition reaction. When the substrate
is asymmetric, regioselectivity is determined by Zaitsevs
rule or through Hofmann elimination if the Carbon with
the most substituted Hydrogen is inaccessible.
The C-H bond is weakened in the rate determining
step and therefore a primary deuterium isotope effect much larger than 1 (commonly 2-6) is observed.
E2 competes with the SN2 reaction mechanism.
E2 mechanism
Scheme 1. E2 reaction mechanism
During the 1920s, Sir Christopher Ingold proposed a
model to explain a peculiar type of chemical reaction;
the E2 mechanism. E2 stands for bimolecular elimination. The reaction involves a one-step mechanism in
which carbon-hydrogen and carbon-halogen bonds break
to form a double bond. C=C Pi bond.
An example of this type of reaction in scheme 1 is the
reaction of isobutylbromide with potassium ethoxide in
ethanol. The reaction products are isobutylene, ethanol
and potassium bromide.
The specics of the reaction are as follows:
2 E1 mechanism
E1 is a model to explain a particular type of chemical
E2 is the rst step of elimination with a single
elimination reaction. E1 stands for unimolecular elimitransition state.
nation and has the following specicities.
Typically undergone by primary substituted alkyl
halides, but is possible with some secondary alkyl
halides.
It is a two-step process of elimination: ionization
and deprotonation.
1
5
Ionization: the carbon-halogen bond breaks to
give a carbocation intermediate.
Deprotonation of the carbocation.
E1 typically takes place with tertiary alkyl halides,
but is possible with some secondary alkyl halides.
REFERENCES
Highly substituted carbocations are more stable than
methyl or primary substituted cations. Such stability
gives time for the two-step E1 mechanism to occur.
If SN1 and E1 pathways are competing, the E1 pathway can be favored by increasing the heat.
The reaction rate is inuenced only by the concen- Specic features : 1 . Rearrangement possible 2 . Indetration of the alkyl halide because carbocation for- pendent of concentration and basicity of base
mation is the slowest step aka rate-determining step.
Therefore rst-order kinetics apply (unimolecular).
Reaction usually occurs in complete absence of base 3 E2 and E1 elimination nal notes
or presence of only a weak base (acidic conditions
and high temperature).
The reaction rate is inuenced by halogen's reactivity,
iodide and bromide being favored. Fluoride is not a good
E1 reactions are in competition with SN1 reactions
leaving group. There is a certain level of competition
because they share a common carbocationic interbetween elimination reaction and nucleophilic substimediate.
tution. More precisely, there are competitions between
A secondary deuterium isotope eect of slightly E2 and SN2 and also between E1 and SN1. Substitution generally predominates and elimination occurs only
larger than 1 (commonly 1 - 1.5) is observed.
during precise circumstances. Generally, elimination is
No antiperiplanar requirement. An example is the favored over substitution when
pyrolysis of a certain sulfonate ester of menthol:
steric hindrance increases
basicity increases
temperature increases
the steric bulk of the base increases (such as in
Potassium tert-butoxide)
E1 elimination Nash 2008, antiperiplanar relationship in blue
the nucleophile is poor
In one study [4] the kinetic isotope eect (KIE) was determined for the gas phase reaction of several alkyl halides
with the chlorate ion. In accordance with an E2 elimination the reaction with t-butyl chloride results in a KIE of
2.3. The methyl chloride reaction (only SN2 possible) on
Accompanied by carbocationic rearrangement reac- the other hand has a KIE of 0.85 consistent with a SN2 reaction because in this reaction type the C-H bonds tighten
tions
in the transition state. The KIEs for the ethyl (0.99) and
isopropyl (1.72) analogues suggest competition between
the two reaction modes.
Only reaction product A results from antiperiplanar elimination, the presence of product
B is an indication that an E1 mechanism is
occurring.[3]
4 See Also
Scheme 2. E1 reaction mechanism
E1cB-elimination reaction
An example in scheme 2 is the reaction of tertbutylbromide with potassium ethoxide in ethanol.
5 References
E1 eliminations happen with highly substituted alkyl
halides due to 2 main reasons.
Highly substituted alkyl halides are bulky, limiting
the room for the E2 one-step mechanism; therefore,
the two-step E1 mechanism is favored.
[1] Organic Syntheses I:185 http://orgsynth.org/orgsyn/pdfs/
CV1P0183.pdf
[2] March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York:
Wiley, ISBN 0-471-85472-7
[3] Nash, J. J.; Leininger, M. A.; Keyes, K. (April
2008). Pyrolysis of Aryl Sulfonate Esters in the
Absence of Solvent: E1 or E2?
A Puzzle for
the Organic Laboratory. Journal of Chemical Education 85 (4): 552. Bibcode:2008JChEd..85..552N.
doi:10.1021/ed085p552.
[4] Stephanie M. Villano, Shuji Kato, and Veronica M. Bierbaum (2006). Deuterium Kinetic Isotope Eects in GasPhase SN2 and E2 Reactions: Comparison of Experiment
and Theory. J. Am. Chem. Soc. 128 (3): 736737.
doi:10.1021/ja057491d. PMID 16417360.
6 TEXT AND IMAGE SOURCES, CONTRIBUTORS, AND LICENSES
Text and image sources, contributors, and licenses
6.1
Text
Elimination reaction Source: http://en.wikipedia.org/wiki/Elimination%20reaction?oldid=636465402 Contributors: Michael Hardy,
Karlwick, RedWolf, Naddy, Hadal, Polychrome, Robodoc.at, D3, Icairns, Sam Hocevar, Shudog, Arcadian, Keenan Pepper, Ceyockey,
LOL, Jfx319, V8rik, Bubbachuck, YurikBot, Person unknown, Esprit15d, GraemeL, Itub, SmackBot, Edgar181, Lovecz, MalafayaBot,
DMacks, Marcipangris, Pedrora, Lottamiata, Briansal, Rieman 82, Thijs!bot, Nonagonal Spider, JAnDbot, MER-C, Sushant gupta, Ovy,
R'n'B, CommonsDelinker, LordAnubisBOT, DorganBot, Mikearmet, Lears Fool, Lamro, OKBot, Anchor Link Bot, ClueBot, DragonBot,
SilvonenBot, Addbot, DOI bot, LaaknorBot, Xerxes b, Luckas-bot, Yobot, Xqbot, Elvim, GrouchoBot, Brandon5485, Shadowjams, Jupiterccnetcom, Citation bot 1, Park4223, Thecurran91, Russot1, TjBot, Mrseanski, EmausBot, Tobraider, Orange Suede Sofa, ChuispastonBot,
Rmashhadi, ClueBot NG, Bibcode Bot, Gladissk, ChrisGualtieri, Physchem 13, Priyanka Sandhu, Monkbot, Owais Khursheed, Mtalley90,
Y-S.Ko and Anonymous: 77
6.2
Images
File:E1-eliminationNash2008.svg Source: http://upload.wikimedia.org/wikipedia/commons/f/fd/E1-eliminationNash2008.svg License:
CC-BY-SA-3.0 Contributors: Transferred from en.wikipedia; transferred to Commons by User:Quadell using CommonsHelper. Original
artist: Original uploader was V8rik at en.wikipedia
File:E1_Elimination_Reaction.png Source: http://upload.wikimedia.org/wikipedia/commons/f/fe/E1_Elimination_Reaction.png License: CC-BY-SA-3.0 Contributors: Originally from en.wikipedia; description page is/was here. Original artist: Original uploader was
V8rik at en.wikipedia
File:E2_Elimination_Reaction.png Source: http://upload.wikimedia.org/wikipedia/commons/d/d6/E2_Elimination_Reaction.png License: CC-BY-SA-3.0 Contributors: Originally from en.wikipedia; description page is/was here. Original artist: Original uploader was
V8rik at en.wikipedia
File:EliminationReactionCyclohexene.svg
Source:
http://upload.wikimedia.org/wikipedia/commons/4/44/
EliminationReactionCyclohexene.svg License: CC-BY-SA-3.0 Contributors: Transferred from en.wikipedia; transferred to Commons by User:Choij using CommonsHelper.
Original artist: V8rik (talk). Original uploader was V8rik at en.wikipedia
File:Wikiquote-logo.svg Source: http://upload.wikimedia.org/wikipedia/commons/f/fa/Wikiquote-logo.svg License: Public domain
Contributors: ? Original artist: ?
6.3
Content license
Creative Commons Attribution-Share Alike 3.0
You might also like
- CHM12 Experiment 5 KineticsDocument15 pagesCHM12 Experiment 5 Kineticsshaam030% (2)
- Asymmetric SynthesisDocument55 pagesAsymmetric Synthesisevsgoud_goud0% (1)
- Elimination ReactionDocument22 pagesElimination ReactionSaidul Islam Rupok100% (1)
- Elimination Reaction NotesDocument10 pagesElimination Reaction Notesseema yadavNo ratings yet
- Practice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersFrom EverandPractice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersNo ratings yet
- Department of Chemical EngineeringDocument12 pagesDepartment of Chemical EngineeringSheikh AliNo ratings yet
- AC 101 Unit 1 Titrimetric AnalysisDocument90 pagesAC 101 Unit 1 Titrimetric AnalysisRishabh Kumar Singh100% (1)
- Cape Chemistry Unit Ii Module I Alcohols and Phenol and Alkenes Worksheet and Revision GuideDocument10 pagesCape Chemistry Unit Ii Module I Alcohols and Phenol and Alkenes Worksheet and Revision GuideAshli GrantNo ratings yet
- Free Radical Substitution and Electrophilic AdditionDocument17 pagesFree Radical Substitution and Electrophilic Additionchicko33No ratings yet
- Organic Reactions and Their MechanismsDocument24 pagesOrganic Reactions and Their Mechanismsankita_friends100No ratings yet
- Chem 242 - Chapters 1&2 PDFDocument30 pagesChem 242 - Chapters 1&2 PDFKhaled AbeedNo ratings yet
- Redox EquilibriaDocument2 pagesRedox Equilibriafunkykid80No ratings yet
- CMT552 4 Electrolyte ConductanceDocument57 pagesCMT552 4 Electrolyte ConductanceAira Ariana100% (1)
- SN1 SN2Document54 pagesSN1 SN2Feby Shyntia AfirantiNo ratings yet
- Aromatic Electrophillic - IIDocument15 pagesAromatic Electrophillic - IInananana100% (2)
- Enzyme and Acid - Base CatalysisDocument64 pagesEnzyme and Acid - Base Catalysisbinseung skzNo ratings yet
- Wagner-Meerwein RearrangementsDocument2 pagesWagner-Meerwein RearrangementsNguyễn Đình SơnNo ratings yet
- Chapter 7 PDFDocument80 pagesChapter 7 PDFBaban BaidyaNo ratings yet
- To Study The Kinetics of Persulphate-Iodide Ion Reaction by Initial Rate Method (Iodine Clock Reaction)Document12 pagesTo Study The Kinetics of Persulphate-Iodide Ion Reaction by Initial Rate Method (Iodine Clock Reaction)Nishika GeraNo ratings yet
- Chemical BondingDocument68 pagesChemical BondingHarsh Tyagi100% (2)
- Elimination ReactionDocument40 pagesElimination ReactionAnkit AnandNo ratings yet
- Finals PhychemDocument3 pagesFinals PhychemniezajanepatnaNo ratings yet
- ATOOCV1 7 0 Aliphatic Electrophilic SubstitutionDocument18 pagesATOOCV1 7 0 Aliphatic Electrophilic SubstitutionVel SankarNo ratings yet
- Complex Reactions: Dr. Rer. Nat. Deni RahmatDocument38 pagesComplex Reactions: Dr. Rer. Nat. Deni Rahmathelenismaya100% (1)
- Alpha Carbon Chemistry - Enols and EnolatesDocument49 pagesAlpha Carbon Chemistry - Enols and EnolatesKuku MandavaNo ratings yet
- E-Z NomenclatureDocument11 pagesE-Z NomenclatureObsa AbrahimNo ratings yet
- Enamines and YlidesDocument18 pagesEnamines and YlidesVijay Pradhan100% (1)
- F325 Acids and PHDocument19 pagesF325 Acids and PHDoc_CrocNo ratings yet
- Debye Huckel Limiting Law of ActivityDocument15 pagesDebye Huckel Limiting Law of ActivitySarad ChaudharyNo ratings yet
- 7 - JEE - Chemistry - Electrochemistry - Transport Number or Transference NumberDocument2 pages7 - JEE - Chemistry - Electrochemistry - Transport Number or Transference NumberGurmehakdeep Billa100% (1)
- Module 1 Lec 2 - THERMODYNAMICS 2nd QTR SY1112 PDFDocument8 pagesModule 1 Lec 2 - THERMODYNAMICS 2nd QTR SY1112 PDFJason JohnsonNo ratings yet
- Types of ElectrolytesDocument95 pagesTypes of ElectrolytesDeepak Sirone100% (4)
- Principles of Neutralization TitrationDocument32 pagesPrinciples of Neutralization TitrationAldwin CantosNo ratings yet
- Organic Reaction MechanismsDocument14 pagesOrganic Reaction MechanismstylerNo ratings yet
- Elimination Reactions Mechanism Lecture NotesDocument17 pagesElimination Reactions Mechanism Lecture NotesveluselvamaniNo ratings yet
- Ionic Bonding - Compounds and PropertiesDocument21 pagesIonic Bonding - Compounds and PropertiesJawaid IqbalNo ratings yet
- Electroanalytical ChemistryDocument4 pagesElectroanalytical ChemistrybelleNo ratings yet
- Aldehydes and KetonesDocument13 pagesAldehydes and Ketonesaleah kimNo ratings yet
- 01 1350977450 79497 PDFDocument83 pages01 1350977450 79497 PDFArya ChowdhuryNo ratings yet
- Green Chemistry ExperimentDocument3 pagesGreen Chemistry ExperimentAnnalisa GiammòNo ratings yet
- Comparing The SN1 and SN2 Reactions - Master Organic ChemistryDocument5 pagesComparing The SN1 and SN2 Reactions - Master Organic Chemistryprince ranaNo ratings yet
- Atomic Structure NotesDocument9 pagesAtomic Structure Notescgao30No ratings yet
- Reaction IntermediateDocument20 pagesReaction IntermediateSiddarth Singh100% (2)
- Unit 4 Rate of Reaction AnswersDocument38 pagesUnit 4 Rate of Reaction Answersareyouthere92No ratings yet
- Pharmaceutical Analysis and Quality AssuranceDocument15 pagesPharmaceutical Analysis and Quality AssuranceSayeeda MohammedNo ratings yet
- Answer KeyDocument6 pagesAnswer KeyMadhavanIceNo ratings yet
- Elimination Reactions of Alkyl HalidesDocument24 pagesElimination Reactions of Alkyl HalidesmaulidyaNo ratings yet
- Qualitative Treatment of Molecular Orbital TheoryDocument27 pagesQualitative Treatment of Molecular Orbital TheoryIfiok Usoro100% (1)
- Colloid Chemistry and Phase Rule 4022146-1: (Essentials of Physical Chemistry - Arun Bahl & B.S. Bahl) Chapter 19 and 22Document51 pagesColloid Chemistry and Phase Rule 4022146-1: (Essentials of Physical Chemistry - Arun Bahl & B.S. Bahl) Chapter 19 and 22Razan khalidNo ratings yet
- Michaelis Menten EquationDocument9 pagesMichaelis Menten Equationsadaf zaidiNo ratings yet
- ASA PPT NOESYDocument15 pagesASA PPT NOESYgovind ashokrao100% (1)
- Electroanalytical ChemistryDocument31 pagesElectroanalytical Chemistryyouni_2005No ratings yet
- Chemistry Unit 2Document8 pagesChemistry Unit 2sashabelleNo ratings yet
- Chemical KineticsDocument49 pagesChemical KineticsS KNo ratings yet
- Aromaticity NotesDocument10 pagesAromaticity NotesVirendra Singh Rajput100% (1)
- Analytical Chemistry 1Document20 pagesAnalytical Chemistry 1Andrew May Ncube100% (1)
- Classification of Organometallic CompoundsDocument28 pagesClassification of Organometallic CompoundsDingetegna GodanaNo ratings yet
- S. NO. Jee-Main+Advance Batch Subject Name of TopicDocument1 pageS. NO. Jee-Main+Advance Batch Subject Name of TopicKrishna BajpaiNo ratings yet
- Lab Report 606 GCDocument7 pagesLab Report 606 GCalyssaswandiNo ratings yet
- Raman Spectroscopy of Few-Quintuple Layer Topological Insulator Bi Se NanoplateletsDocument8 pagesRaman Spectroscopy of Few-Quintuple Layer Topological Insulator Bi Se Nanoplateletssena4kulaks4zNo ratings yet
- Drum DataDocument3 pagesDrum DataAnonymous XV9LfaMmNo ratings yet
- What Is Atomic TheoryDocument2 pagesWhat Is Atomic TheoryAyessa AnchetaNo ratings yet
- Electrode Oil Spectrometer HOPUDocument4 pagesElectrode Oil Spectrometer HOPUSamir AjiNo ratings yet
- Nickel and Its AlloysDocument162 pagesNickel and Its AlloysAnonymous TI2bUT100% (1)
- Chemical Properties and Charcoal Quality of Five Wood Species From West KalimantanDocument14 pagesChemical Properties and Charcoal Quality of Five Wood Species From West KalimantanIsmiraisa AzariaNo ratings yet
- JSW Steel MTCDocument5 pagesJSW Steel MTCNelson 2428No ratings yet
- 3 Main Types of StarDocument8 pages3 Main Types of StarLeslie GandaNo ratings yet
- HC Verma Ray OpticsDocument25 pagesHC Verma Ray Opticsloke472008No ratings yet
- Gravitational and Electric FieldsDocument70 pagesGravitational and Electric Fieldswarren palmerNo ratings yet
- Design Vacuum UnitsDocument6 pagesDesign Vacuum UnitsAmit YadavNo ratings yet
- Fire and Explosion of LPG TankDocument14 pagesFire and Explosion of LPG TankRhama WijayaNo ratings yet
- Engineering Chemistry A TextbookDocument217 pagesEngineering Chemistry A Textbookpankaj vashisht100% (2)
- Boiling PDFDocument11 pagesBoiling PDFRahul Kotadiya100% (1)
- Technical Report On SRV and Safety Fittings For TBI PDFDocument31 pagesTechnical Report On SRV and Safety Fittings For TBI PDFashraf ahmadNo ratings yet
- BIO002 - Introductory Biology Lecture 2 and 3 AY2018-2019Document10 pagesBIO002 - Introductory Biology Lecture 2 and 3 AY2018-2019feviola tNo ratings yet
- Gauss On Celestial MechanicsDocument418 pagesGauss On Celestial MechanicsIMANUL100% (3)
- Seminar Report PDFDocument22 pagesSeminar Report PDFRajani GandhaNo ratings yet
- SAMPLE PAPER 27 2024 - 25Document12 pagesSAMPLE PAPER 27 2024 - 25Bhavana Surana 7ENo ratings yet
- Unit-I Electrochemistry & CorrosionDocument38 pagesUnit-I Electrochemistry & CorrosionNitish ReddyNo ratings yet
- Safety Data Sheet Nitrogen, CompressedDocument12 pagesSafety Data Sheet Nitrogen, CompressedKafi Mahmood NahinNo ratings yet
- Engine Oil Viscosity and SAE GradesDocument2 pagesEngine Oil Viscosity and SAE Gradeschandra BandaraNo ratings yet
- Updated Syllabus For Neet 2025 by Neetxrt - WatermarkDocument14 pagesUpdated Syllabus For Neet 2025 by Neetxrt - Watermarknppatil1527No ratings yet
- Flat Earth No Space TravelDocument6 pagesFlat Earth No Space TravelAnthony100% (2)
- Fiitjee: Admission TestDocument13 pagesFiitjee: Admission TestPBNo ratings yet
- Functionality Description - Kovac and RadakDocument2 pagesFunctionality Description - Kovac and RadakFilip KovacNo ratings yet
- Course Objectives:: Refrigeration and Air-ConditioningDocument5 pagesCourse Objectives:: Refrigeration and Air-ConditioningLeo BoyNo ratings yet
- Test SciDocument7 pagesTest SciMary Ann MariquitNo ratings yet