DRUG STUDY - Ferrous Sulfate
DRUG STUDY - Ferrous Sulfate
Uploaded by
Siergs Smith GervacioCopyright:
Available Formats
DRUG STUDY - Ferrous Sulfate
DRUG STUDY - Ferrous Sulfate
Uploaded by
Siergs Smith GervacioOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
DRUG STUDY - Ferrous Sulfate
DRUG STUDY - Ferrous Sulfate
Uploaded by
Siergs Smith GervacioCopyright:
Available Formats
Patients Name:
Aida Paco
Age:
57 years old
Female
Diagnosis:
AUB-M, Endometrioid Carcinoma
Sex:
Physician:
Dr. Laplana, Charlene
Date of Admission:
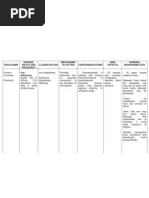
Name of Drug
Classification
Mechanism of Action
Indication
Generic Name: Ferrous Sulfate + MV
Pharmacologic Class:
Chemical:
Trade/Brand Name:
Hematinic
Elevates the serum iron concentration which then
Feosol
Therapeutic:
helps to form High or trapped in the
General Indication:
Fer Iron
Oral Supplement
reticuloendothelial cells for storage and eventual
Fer-Gen-Sol
Pregnancy Risk Category:
conversion to a usable form of iron.
Fer-Iron
Category A
Therapeutic:
Ferrin
Source:
Necessary for effective erythropoiesis
Patients Dose: O.9 mL OD
NDH p.571-574
Pharmacokinetics:
Maximum Dose: 15 mL
Minimum Dose: 1 to 2 mL
Contents:
Iron + Multivitamins
Availability:
Rx and/or OTC
Route(s) of Administration:
PO
Source:
NDH p.571-574
November 10, 2015
Route:
PO
Onset:
4 days
Source:
NDH p.571-574
Prevention and treatment of
iron deficiency anemias.
Dietary supplement for iron.
Patients Indication:
Peak:
7-10 days
Dietary supplement for iron.
Duration:
2-4
months
Drug Half-Life: 6 hours
Source
NDH p.571-574
Height:
5 ft
Weight:
45 kg
Contraindication
Contraindication:
Hypersensitivity
Severe hypotension.
Ulcerated colon
Haemolytic anemia
PUD
Precautions:
Elderly patients
iron overload disorder
patients with liver problems
patients with stomach/intestinal problems
Drug Interactions:
antibiotics
bisphosphonates
levodopa,
methyldopa,
thyroid replacement drugs
Source:
NDH p.571-574
Side Effects
Dizziness
N&V
Nasal Congestion
Dyspnea
Hypotension
CHF
MI
Muscle cramps
Flushing
Staining of teeth
Black stools
Source:
NDH p.571-574
Nursing Responsibilities
Before:
Observe the 10Rs of drug administration
Ask for drug allergies
Administer the prescribed dosage
Check the doctors order
Teach SO to let her child take FeSO4 on an empty stomach
During:
Caution patient to make position changes slowly to minimize
orthostatic hypotension.
Instruct patient to avoid concurrent use of alcohol or OTC
medicine without consulting the physician.
Inform patient that defecating black stools is normal
Let the patient take ferrous sulphate with straw to avoid
staining of teeth
Dont take FeSO4 in large amounts of longer than
recommended
After:
Advise patient to consult physician if irregular heartbeat,
dyspnea, swelling of hands and feet and hypotension occurs.
Inform patient that angina attacks may occur 30 min. after
administration due reflex tachycardia. (happens rarely)
Encourage patient to comply with additional intervention for
hypertension like proper diet, regular exercise, and lifestyle
changes and stress management. (for patients with HPN)
Seek medical attention of adverse reactions occur
Documentation
Source:
NDH p.571-574
You might also like
- Professional Adjustment Practice QuestionDocument41 pagesProfessional Adjustment Practice QuestionSiergs Smith Gervacio100% (1)
- Iron Supplement - During Pregnancy, Requirements For Iron IncreaseDocument1 pageIron Supplement - During Pregnancy, Requirements For Iron Increasegeorgeloto12No ratings yet
- Food and Beverage DepartmentDocument11 pagesFood and Beverage DepartmentSarah AucklandNo ratings yet
- M 09 05Document254 pagesM 09 05D PNo ratings yet
- OxytocinDocument2 pagesOxytocinJoanne Kaye TaylorNo ratings yet
- Fe SO4Document3 pagesFe SO4CarmellaDawnNo ratings yet
- Drug SDocument2 pagesDrug SJane CasiquinNo ratings yet
- Name of Drug Mechanism of Action Indications and Contraindication Adverse Effect Nursing ConsiderationDocument2 pagesName of Drug Mechanism of Action Indications and Contraindication Adverse Effect Nursing ConsiderationNicole CalpoturaNo ratings yet
- Drug StudyDocument3 pagesDrug StudyNiño Robert RodriguezNo ratings yet
- ParacetamolDocument2 pagesParacetamolBlesyl Sison Mabano100% (1)
- Ferrous SulfateDocument2 pagesFerrous SulfateJoesineNo ratings yet
- DRUG Ferrous SulfateDocument1 pageDRUG Ferrous SulfateLovelaine Busto AlaguiaNo ratings yet
- Name of Drug Dosage/Frequency/ Timing/Route Mechanism of Action Indication Contraindication Adverse Effect Nursing ResponsibilitiesDocument2 pagesName of Drug Dosage/Frequency/ Timing/Route Mechanism of Action Indication Contraindication Adverse Effect Nursing ResponsibilitieskyleNo ratings yet
- Drug Study 1 Ferrous SulfateDocument2 pagesDrug Study 1 Ferrous SulfateKrizzia Mae ColladoNo ratings yet
- Drug Study Ciprofloxacin QuinosynDocument3 pagesDrug Study Ciprofloxacin QuinosynEmmanuel Margate100% (1)
- Sodium BicarbonateDocument1 pageSodium BicarbonateALbinong VelascoNo ratings yet
- Drug Mechanis MOF Action Indicatio N Contraindicatio N Side Effects Adverse Effects Nursing Responsibilit YDocument1 pageDrug Mechanis MOF Action Indicatio N Contraindicatio N Side Effects Adverse Effects Nursing Responsibilit YNica RodriguezNo ratings yet
- CEFOTAXIMEDocument3 pagesCEFOTAXIMEChoox PriiNo ratings yet
- Drug Study Delivery RoomDocument7 pagesDrug Study Delivery RoomkhleeoNo ratings yet
- Stugeron® TabletsDocument3 pagesStugeron® TabletsmahgadNo ratings yet
- Ferrous Sulfate: o o o o o o oDocument5 pagesFerrous Sulfate: o o o o o o oLelanie Japitana100% (1)
- Duavent Drug StudyDocument2 pagesDuavent Drug StudyNicole cuencosNo ratings yet
- Drug StudyDocument4 pagesDrug StudyJan DeeNo ratings yet
- Omeprazole: (Oh Me' Pray Zol)Document3 pagesOmeprazole: (Oh Me' Pray Zol)Athea MelosantosNo ratings yet
- Amoxicillin TrihydrateDocument1 pageAmoxicillin TrihydrateHoney Que BullivantNo ratings yet
- Drug Study Duavent.Document1 pageDrug Study Duavent.Clariss AlotaNo ratings yet
- Drug Study Quinine SulfateDocument7 pagesDrug Study Quinine SulfateKathlyn_Matic_6376No ratings yet
- Drug Study Vitamin C + ZincDocument2 pagesDrug Study Vitamin C + ZincKrizzia FosterNo ratings yet
- Ferrous Sulfate - Drug StudyDocument3 pagesFerrous Sulfate - Drug StudyElla Musk100% (1)
- OB Drug Study - Mefenamic AcidDocument2 pagesOB Drug Study - Mefenamic AcidJustin Ancog100% (1)
- IrbesartanDocument3 pagesIrbesartanapi-3797941No ratings yet
- Drug Study - Cimetidine (Tagamet)Document3 pagesDrug Study - Cimetidine (Tagamet)mikErlh100% (3)
- Drug Study DRDocument6 pagesDrug Study DRBheigh Lomitao AlbueraNo ratings yet
- Drug StudyDocument1 pageDrug Studyzjoshuac100% (1)
- INDOMETHACINDocument1 pageINDOMETHACINRPh Krishna Chandra JagritNo ratings yet
- A Drug Study On Evening Primrose OilDocument5 pagesA Drug Study On Evening Primrose OilAlexis Khalyl Y. MontejoNo ratings yet
- ZafirlukastDocument3 pagesZafirlukastapi-379794167% (3)
- CiticolineDocument1 pageCiticolineHarvey BanagNo ratings yet
- GUAIFENESINDocument1 pageGUAIFENESINAngel CatalanNo ratings yet
- Patient M. G Drug 1 - Ob MaxDocument5 pagesPatient M. G Drug 1 - Ob MaxGrace MellaineNo ratings yet
- Ampicillin 2Document1 pageAmpicillin 2Kristine YoungNo ratings yet
- Pravastatin SodiumDocument3 pagesPravastatin Sodiumapi-3797941No ratings yet
- TergecefDocument2 pagesTergecefianecunar100% (3)
- Drug Study FinalDocument5 pagesDrug Study FinalJackie Ann Marie DapatNo ratings yet
- Calcium Gluconate Drug SummDocument1 pageCalcium Gluconate Drug SummAminah Yue100% (1)
- Drug Study Ferrous SulfateDocument2 pagesDrug Study Ferrous SulfateBunnie AlphaNo ratings yet
- Drug Study AzithromycinDocument2 pagesDrug Study AzithromycinYamete KudasaiNo ratings yet
- Drug Study Ferrous SulfateDocument2 pagesDrug Study Ferrous SulfatePauline AnesNo ratings yet
- DORMICUMDocument1 pageDORMICUMArian Rose100% (1)
- Drug Study AGEDocument5 pagesDrug Study AGEAna Mae ArellanoNo ratings yet
- DRUG STUDY OXYTOCIN, METHERGINE EtcDocument9 pagesDRUG STUDY OXYTOCIN, METHERGINE EtcPatricia Reese YutiamcoNo ratings yet
- Ferrous SulfateDocument2 pagesFerrous SulfateChris Jake GonzalesNo ratings yet
- GENTAMICINDocument3 pagesGENTAMICINjacquejackieNo ratings yet
- AeknilDocument2 pagesAekniljaycey24No ratings yet
- Drug Study QIDocument8 pagesDrug Study QImaeDonitaNo ratings yet
- Ferlin PDFDocument1 pageFerlin PDFRomeo ReyesNo ratings yet
- Drug Study: Name of PatientDocument1 pageDrug Study: Name of PatientKaloy KamaoNo ratings yet
- DRUG STUDY (Erythromycin)Document3 pagesDRUG STUDY (Erythromycin)Avianna CalliopeNo ratings yet
- OB Drug StudyDocument12 pagesOB Drug StudyCj AttoNo ratings yet
- Tamsulosin - Drug Information - UpToDateDocument23 pagesTamsulosin - Drug Information - UpToDateGénesis GabrielaNo ratings yet
- Comparison of Efficacy & Safety of Iron Polymaltose Complex & Ferrous Ascorbate With Ferrous Sulphate in Pregnant Women With Iron-Deficiency AnaemiaDocument7 pagesComparison of Efficacy & Safety of Iron Polymaltose Complex & Ferrous Ascorbate With Ferrous Sulphate in Pregnant Women With Iron-Deficiency AnaemiaNimesh ModiNo ratings yet
- S 0007114510005490 ADocument8 pagesS 0007114510005490 ABojan PavlovićNo ratings yet
- Prospective Study To Evaluate Oral Iron Preparations in Antenatal Women at A Tertiary Care HospitalDocument4 pagesProspective Study To Evaluate Oral Iron Preparations in Antenatal Women at A Tertiary Care HospitalAF KoasNo ratings yet
- Comprehensive Exam 1Document19 pagesComprehensive Exam 1karenkaren09100% (2)
- DRUG STUDY - MetoprololDocument1 pageDRUG STUDY - MetoprololSiergs Smith Gervacio100% (2)
- Drug Study - CelecoxibDocument1 pageDrug Study - CelecoxibSiergs Smith GervacioNo ratings yet
- DRUG STUDY - Vit B ComplexDocument2 pagesDRUG STUDY - Vit B ComplexSiergs Smith Gervacio78% (9)
- Drug Study - TramadolDocument1 pageDrug Study - TramadolSiergs Smith GervacioNo ratings yet
- Drug Study - CefuroximeDocument2 pagesDrug Study - CefuroximeSiergs Smith GervacioNo ratings yet
- Drug Study - DigoxinDocument1 pageDrug Study - DigoxinSiergs Smith Gervacio100% (2)
- Drug Study - CefoxitinDocument1 pageDrug Study - CefoxitinSiergs Smith Gervacio100% (6)
- DRUG STUDY - Calcium GluconateDocument2 pagesDRUG STUDY - Calcium GluconateSiergs Smith Gervacio100% (2)
- Placenta Previa Case StudyDocument59 pagesPlacenta Previa Case StudySiergs Smith GervacioNo ratings yet
- Practice Questions NLEDocument418 pagesPractice Questions NLESiergs Smith Gervacio100% (2)
- Acute GastroenteritisDocument28 pagesAcute GastroenteritisSiergs Smith GervacioNo ratings yet
- Practice Questions NLEDocument418 pagesPractice Questions NLESiergs Smith Gervacio100% (2)
- Emergency DrugsDocument26 pagesEmergency DrugsSiergs Smith Gervacio100% (1)
- NCP - Risk For InfectionDocument3 pagesNCP - Risk For InfectionSiergs Smith Gervacio100% (3)
- Vit and MineralsDocument12 pagesVit and MineralsSiergs Smith GervacioNo ratings yet
- Surgical EquipmentsDocument6 pagesSurgical EquipmentsSiergs Smith GervacioNo ratings yet
- Ectopic Pregnancy PathophysiologyDocument2 pagesEctopic Pregnancy PathophysiologySiergs Smith GervacioNo ratings yet
- Ectopic Pregnancy PathophysiologyDocument2 pagesEctopic Pregnancy PathophysiologySiergs Smith GervacioNo ratings yet
- NCP FormDocument4 pagesNCP FormSiergs Smith GervacioNo ratings yet
- Income TaxCalculator SRS Ver3.0Document8 pagesIncome TaxCalculator SRS Ver3.0rahul_xxxruNo ratings yet
- (Pmls1) Lesson 5 Medical Technology EducationDocument2 pages(Pmls1) Lesson 5 Medical Technology Educationteresa.catudayNo ratings yet
- Year 2 Literacy Homework WorksheetsDocument6 pagesYear 2 Literacy Homework Worksheetsafeufjxsq100% (1)
- Instant Download Haig's Enemy: Crown Prince Rupprecht and Germany's War On The Western Front Jonathan Boff PDF All ChapterDocument64 pagesInstant Download Haig's Enemy: Crown Prince Rupprecht and Germany's War On The Western Front Jonathan Boff PDF All Chaptergdggxjieben35100% (4)
- Role of NGO in WelfareDocument4 pagesRole of NGO in WelfareUmesh Gadekar0% (1)
- 2023 Paper 1ADocument7 pages2023 Paper 1AabubakarNo ratings yet
- Chapter 3Document32 pagesChapter 3api-394738731No ratings yet
- Volume GANDocument12 pagesVolume GANFei YinNo ratings yet
- Sigma-Aldrich: Material Safety Data SheetDocument6 pagesSigma-Aldrich: Material Safety Data SheetkavsivamNo ratings yet
- Machine Learning Is Fun PDFDocument16 pagesMachine Learning Is Fun PDFmalliwi0% (1)
- A Journey Through The History of ComputersDocument3 pagesA Journey Through The History of ComputersAngelo RuizNo ratings yet
- Sae J109-2014Document4 pagesSae J109-2014Marcos RosenbergNo ratings yet
- AirbagDocument26 pagesAirbagGourab Saha100% (2)
- Product Guide - April PDFDocument75 pagesProduct Guide - April PDFPunith KumarNo ratings yet
- Download Working for Yourself: Law & Taxes for Independent Contractors, Freelancers & Gig Workers of All Types Stephen Fishman ebook All Chapters PDFDocument40 pagesDownload Working for Yourself: Law & Taxes for Independent Contractors, Freelancers & Gig Workers of All Types Stephen Fishman ebook All Chapters PDFtillygresl4cNo ratings yet
- FRM 2018 Lobs PDFDocument52 pagesFRM 2018 Lobs PDFAnonymous gtP37gHONo ratings yet
- Sylphy: Photo May Vary From Actual UnitDocument8 pagesSylphy: Photo May Vary From Actual UnitLester Mark LignaNo ratings yet
- Econ368 Syllabus S19.1.10Document8 pagesEcon368 Syllabus S19.1.10blakeNo ratings yet
- Commercialized Hospital Information Systems World Wide WebDocument1 pageCommercialized Hospital Information Systems World Wide WebFRANCESCA GABRIEL DOMINGONo ratings yet
- Mark-Up Mark-On MarkdownDocument41 pagesMark-Up Mark-On MarkdownravenbaklasupotNo ratings yet
- Swimming Pool Evaporation Loss Calculation VERY IMPORTANTDocument5 pagesSwimming Pool Evaporation Loss Calculation VERY IMPORTANTonspsnonsNo ratings yet
- SOSOHbyUGMD MJsectionDocument18 pagesSOSOHbyUGMD MJsectionSushant RathiNo ratings yet
- Marketing Strategies of Coca-Cola 2019031Document70 pagesMarketing Strategies of Coca-Cola 2019031Saili ChodankarNo ratings yet
- High Vacuum Storage ContainersDocument2 pagesHigh Vacuum Storage ContainerspcbstepNo ratings yet
- CAP Medal of ValorDocument4 pagesCAP Medal of ValorCAP History LibraryNo ratings yet
- Gastech 19 Sales BrochureDocument24 pagesGastech 19 Sales BrochureAnmol singhNo ratings yet
- 094 Munsch Mechanuically Sealed NP B BrochureDocument6 pages094 Munsch Mechanuically Sealed NP B BrochureAboody WahdainNo ratings yet
- Fundamentals of Human Resource Management: Compensation and Total RewardsDocument30 pagesFundamentals of Human Resource Management: Compensation and Total Rewardspapul884No ratings yet