Ruby Laser
Uploaded by
Vignesh AukRuby Laser
Uploaded by
Vignesh AukRuby LASER:
The first working laser was built in 1960 by Maiman, using a ruby crytal and so
called the Ruby laser.
Ruby belongs to the family of gems consisting of Al2O3 with various types of
impurities. For example pink Ruby contains 0.05% Cr atoms.
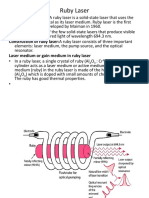
Schematic diagram of ruby laser
Construction of Ruby Laser
The ruby lazer consists of a ruby rod which is made of chromium doped ruby
material. At the opposite ends of this rod there are two silver polished mirrors.
Whose one is fully polished and other is partially polished. A spring is attached to
the rod with fully polished end for adjustment of wave length of the lazer light.
Around the ruby rod a flash light is kept for the pump input. The whole assembly is
kept in the glass tube. Around the neck of the glass tube the R.F source and
switching control is designed in order to switch on and off the flash light for
desired intervals.
Operation of Ruby Laser:
When we switch on the circuit the R.F operates. As a result the flash of light is
obtained around the ruby rod. this flash causes the electrons within ruby rod to
move from lower energy band towards higher energy band. The population
inversion take place at high energy band and electrons starts back to travel
towards the lower energy band. During this movement the electron emits the laser
light . This emitted light travels between the two mirrors where cross reflection
takes place of this light. The stimulated lazer light now escapes from partially
polished mirror in shape of laser beam.
The spring attached with the fully polished mirror is used to adjust the wave
length equal to /2 of lazer light for obtimum lazer beam. The switching control
of the R.F source is used to switch on and off the flash light so that excessive heat
should not be generated due to very high frequency of the movement of the
electron.
Energy Level Diagram for Ruby Laser
The above three level energy diagram show that in ruby lasers the absorption
occurs in a rather broad range in the green part of the spectrum. This makes raise
the electrons from ground state E1 to the band of level E3 higher than E1. At
E3 these excited levels are highly unstable and so the electrons decays rapidly to
the level of E2. This transition occurs with energy difference (E1 E2) given up as
heat (radiation less transmission). The level E2 is very important for stimulated
emission process and is known as Meta stable state. Electrons in this level have an
average life time of about 5m.s before they fall to ground state. After this the
population inversion can be established between E2 and E1. The population
inversion is obtained by optical pumping of the ruby rod with a flash lamp. A
common type of the flash lamp is a glass tube wrapped around the ruby rod and
filled with xenon gas. When the flash lamp intensity becomes large enough to
create population inversion, then stimulated emission from the Meta stable level
to the ground level occurs which result in the laser output. Once the population
inversion begins, the Meta stable level is depopulated very quickly. Thus the laser
output consists of an intense spike lasting from a few Nano sec to sec. after
stimulated emission spike, population inversion builds up again and a 2nd spike
results. This process continues as long as the flash lamp intensity is enough to
create the population inversion.
Advantages of Ruby Lasers
From cost point of view, the ruby lasers are economical.
Beam diameter of the ruby laser is comparatively less than CO2 gas lasers.
Output power of Ruby laser is not as less as in He-Ne gas lasers.
Since the ruby is in solid form therefore there is no chance of wasting
material of active medium.
Construction and function of ruby laser is self explanatory.
Disadvantages of Ruby Laser
In ruby lasers no significant stimulated emission occurs, until at least half of
the ground state electrons have been excited to the Meta stable state.
Efficiency of ruby laser is comparatively low.
Optical cavity of ruby laser is short as compared to other lasers, which may
be considered a disadvantage.
The broad categories of lasers are:
(i) Optically Pumped Solid-State Lasers
(ii) Liquid (Dye) Lasers
(iii) Gas Lasers
(iv) Semiconductor Lasers
(v) Free Electron Lasers
(vi) X-ray Lasers, and
(vii) Chemical Lasers
Medical applications of Laser
Noninvasive tissue characterization to replace or guide physical biopsy, e.g.
early diagnosis of lung cancer
Most of the eyes optical power is provided by refraction at the air/cornea
interface. In myopia (or near-sightedness) the shape is such that a focused
image is not produced on the retina. This can be corrected with glasses or
contact lenses, or by reshaping the cornea. Originally this was done
surgically. An alternative strategy is to ablate tissue in a very controlled
fashion using a pulsed laser.
Use chemical reactions initiated by light absorption to kill cells. Original
application in oncology but is applicable to other diseases, including age-
related macular degeneration caused by a proliferation of new blood vessels
in the retina
Minimally invasive method to pulverize kidney stones so that they can be
eliminated. Optical fiber inserted through urethra and ureter (or
transcutaneously) and placed near stone under endoscopic visualization.
You might also like
- Acoustic Tractor Beam: 35 Steps (With Pictures) PDFNo ratings yetAcoustic Tractor Beam: 35 Steps (With Pictures) PDF38 pages
- Fifth State of Matter - Used To Make New Type of SuperconductorNo ratings yetFifth State of Matter - Used To Make New Type of Superconductor4 pages
- Central Machinery Model 93212 Manual in English0% (1)Central Machinery Model 93212 Manual in English37 pages
- A Seminar Report ON Grinding: Submitted by Rajshree B. Tech (Mechanical Engineering) Third Year Roll No: 160180104039No ratings yetA Seminar Report ON Grinding: Submitted by Rajshree B. Tech (Mechanical Engineering) Third Year Roll No: 16018010403921 pages
- MB20-200 LMSP-DP STD en Glovebox Manual - MBraunNo ratings yetMB20-200 LMSP-DP STD en Glovebox Manual - MBraun182 pages
- A New Unity Power Factor Quasi-Resonant Induction Heater PDF100% (1)A New Unity Power Factor Quasi-Resonant Induction Heater PDF225 pages
- Machining Operations and Machining ToolsNo ratings yetMachining Operations and Machining Tools93 pages
- AM Receiver: Home Analysis Help Media Links PracticalNo ratings yetAM Receiver: Home Analysis Help Media Links Practical10 pages
- Magneto Hydro Dynamic Power Generation MHD100% (1)Magneto Hydro Dynamic Power Generation MHD23 pages
- Teacher: MR - Seale Student: Destineé JonesNo ratings yetTeacher: MR - Seale Student: Destineé Jones7 pages
- Precision Bearing House, Industrial AutomationNo ratings yetPrecision Bearing House, Industrial Automation244 pages
- Product Realization by Manufacturing: Lab ManualNo ratings yetProduct Realization by Manufacturing: Lab Manual75 pages
- Engineer's Mini-Notebook - Communications ProjectsNo ratings yetEngineer's Mini-Notebook - Communications Projects26 pages
- 30 KVA Induction Heater - 8 Steps (With Pictures) - Instructables100% (3)30 KVA Induction Heater - 8 Steps (With Pictures) - Instructables1 page
- Spring Design: Prepared and Presented By: Manoj AdhikariNo ratings yetSpring Design: Prepared and Presented By: Manoj Adhikari21 pages
- 1626604993-1-10 (1) - 1-10-Pages-1-MergedNo ratings yet1626604993-1-10 (1) - 1-10-Pages-1-Merged9 pages
- Import Tarif: Not Elsewhere Specified or IncludedNo ratings yetImport Tarif: Not Elsewhere Specified or Included8 pages
- Climate Change - A Silent Threat To HumanityNo ratings yetClimate Change - A Silent Threat To Humanity8 pages
- Study Plan B.Sc. Telecommunications: Faculty of Electronics and Information TechnologyNo ratings yetStudy Plan B.Sc. Telecommunications: Faculty of Electronics and Information Technology2 pages
- Allama Iqbal Open University, Islamabad (Department of Computer Science) WarningNo ratings yetAllama Iqbal Open University, Islamabad (Department of Computer Science) Warning4 pages
- Bur 87634742NA - J.C.M SV212, SV216 Tier 3No ratings yetBur 87634742NA - J.C.M SV212, SV216 Tier 348 pages
- User Manual HPS 70009000 Series Single Phase Grid Tied Photovoltaic InverterNo ratings yetUser Manual HPS 70009000 Series Single Phase Grid Tied Photovoltaic Inverter33 pages
- Design of A Low Cost BPSK Modulator/demodulator For A Practical Illustration of Digital ModulationsNo ratings yetDesign of A Low Cost BPSK Modulator/demodulator For A Practical Illustration of Digital Modulations7 pages
- Masterys Ip+: Installation and Operating ManualNo ratings yetMasterys Ip+: Installation and Operating Manual72 pages
- Course Schedule: SEDA Malaysia Grid-Connected Photovoltaic (PV) Systems Design CourseNo ratings yetCourse Schedule: SEDA Malaysia Grid-Connected Photovoltaic (PV) Systems Design Course3 pages
- Characteristics of Instruments and Measurement SystemsNo ratings yetCharacteristics of Instruments and Measurement Systems23 pages
- SERIES 17000, 18000 THERMOSWITCH Temperature Controllers Series 11000, 80016 Protective WellsNo ratings yetSERIES 17000, 18000 THERMOSWITCH Temperature Controllers Series 11000, 80016 Protective Wells7 pages