Redox Reactions and Balancing Using Oxidation Number & Nfactor
Uploaded by
RSLRedox Reactions and Balancing Using Oxidation Number & Nfactor
Uploaded by
RSLAllenBengaluru_Chemistry_VSK_10.
B2_Redox Tuesday, 12 December 2017
Redox Reactions
1. Assign oxidation number to the underline elements in each of the following species:
(a) NaH2PO4 (b) CrO2Cl2 (c) H4P2O7 (d) K2MnO4
(e) CaO2 (f) H2S2O7 (g) KAl(SO4)2.12H2O (h) NaBH4
(i) NH2OH (j) (CH3)2SO (k) [Cr(NH3)6]Cl3 (l) K3[Fe(CN)6]
(m) KIO3 (n) RuO4 (o) K2TaF7 (p) C in diamond
2. Identify the oxidizing and reducing agents for each of the following reactions:
(a) 2AgBr (s) + C6H6O2 (aq) 2HBr (aq) + C6H4O2(aq)
(b) HCHO(l) + 2[Ag(NH3)2]+ (aq) + 3OH (aq) 2Ag(s) + HCOO(aq) + 4NH3(aq) + 2H2O(l)
(c) N2H4(l) + 2H2O2(l) N2(g) + 4H2O(l)
(d) Pb(s) + PbO2(s) + 2H2SO4(aq) 2PbSO4(s) + 2H2O(l)
(e) Sn2+ + 2Hg2+
Hg 22 + + Sn4+
(f) 2Cu2+ + 4I
2CuI + I2
(g) 2Cu2O(s) + Cu2 S(s)
6Cu(s) + SO2 (g)
(h) NaOH + Cl2
NaCl + NaClO + H2O
3. How many moles of electrons are involved in balancing the following redox processes?
(a) H2S + NO3 S + NO
(b) Mn(OH)2 + H2O2 MnO2 + 2H2O
(c) Cr2O7 + Fe2+ + C2O42- Cr3+ + Fe3+ + CO2 + H2O
(d) Br2 + OH BrO3 + Br
4. Match the following
ListI [Compound] ListII [Oxidation state of N]
I. KNO3 (A) 1/3
II. HNO2 (B) 3
III. NH4Cl (C) 0
IV. NaN3 (D) +3
(E) +5
5. One of the following oxides does not react with molecular oxygen: NO, N2O, SO2, SO3 and P4O6. Based on oxidation
numbers, which one is it? Explain. [No. of valence e: N=5, P=5, O=6, S=6]
6. Nitric acid is a strong oxidizing agent. When nitric acid reacts with a strong reducing agent such as zinc metal, which of
the following species is least likely to be produced: N2O, NO, NO2, N2O4, N2O5, NH4 . Explain why.
Page 1 of 2
You might also like
- webcontent_145_511_2_p_Block_Elements_20190918124823No ratings yetwebcontent_145_511_2_p_Block_Elements_2019091812482319 pages
- Soluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and DNo ratings yetSoluble Insoluble 6. Ca (NO 3. K Soluble Soluble: Follow This Format For Question B, C and D4 pages
- Redox Reaction-Important Question and Assignments.No ratings yetRedox Reaction-Important Question and Assignments.3 pages
- Topic 9 Redox Booklet C ANSWERS 2014 (Amended Sept 2015)No ratings yetTopic 9 Redox Booklet C ANSWERS 2014 (Amended Sept 2015)39 pages
- Microsoft Word - Chapter 20 Worksheet RedoxNo ratings yetMicrosoft Word - Chapter 20 Worksheet Redox4 pages
- P Block Elements Group 15 16 Bounce Back One ShotNo ratings yetP Block Elements Group 15 16 Bounce Back One Shot147 pages
- XI Chemistry Open Book Test (Chap # 12 Electrochemistry)No ratings yetXI Chemistry Open Book Test (Chap # 12 Electrochemistry)2 pages
- 1WS Grade 10 IG Chemistry 24-25 - Reduction and OxidationNo ratings yet1WS Grade 10 IG Chemistry 24-25 - Reduction and Oxidation3 pages
- Chemsheets A2 1076 Electrochemistry BookletNo ratings yetChemsheets A2 1076 Electrochemistry Booklet20 pages
- Redox Reactions Oxidation Number: H S O Alcl O Fe Mno CuNo ratings yetRedox Reactions Oxidation Number: H S O Alcl O Fe Mno Cu2 pages
- Stoichiometry-Sheet: 2 (Balancing of Reactions) : Level - 1 1. 1. 2. 3. 4. 5. 6. 7No ratings yetStoichiometry-Sheet: 2 (Balancing of Reactions) : Level - 1 1. 1. 2. 3. 4. 5. 6. 72 pages
- Chemistry - 27 Jan Shift-2 JEE Main 2024 (Session 1)No ratings yetChemistry - 27 Jan Shift-2 JEE Main 2024 (Session 1)7 pages
- Chemistry_27 Jan Shift-2 JEE Main 2024 (Session 1)_250125_180601No ratings yetChemistry_27 Jan Shift-2 JEE Main 2024 (Session 1)_250125_1806017 pages
- 6581a67752684600186611f7_##_Salt Analysis Practice SheetNo ratings yet6581a67752684600186611f7_##_Salt Analysis Practice Sheet3 pages
- NCERT Solutions Class 11 Chemistry Chapter 8 Redox ReactionNo ratings yetNCERT Solutions Class 11 Chemistry Chapter 8 Redox Reaction26 pages
- chem_reactions-practice-problems_2013-03-01_6900 answer key -QBLC checked correctNo ratings yetchem_reactions-practice-problems_2013-03-01_6900 answer key -QBLC checked correct10 pages
- Balancing Chemical Equations: Things You Should Know (Questions and Answers)From EverandBalancing Chemical Equations: Things You Should Know (Questions and Answers)No ratings yet
- Carbon Monoxide or Carbonyl: MO DescriptionNo ratings yetCarbon Monoxide or Carbonyl: MO Description3 pages
- Colloidal Gold. Part I: Historical and Preparative Aspects, Morphology and Structure100% (1)Colloidal Gold. Part I: Historical and Preparative Aspects, Morphology and Structure6 pages
- Epoxides Ring-Opening - Chemistry LibreTextsNo ratings yetEpoxides Ring-Opening - Chemistry LibreTexts3 pages
- Turkevich1985 Article ColloidalGoldPartII PDFNo ratings yetTurkevich1985 Article ColloidalGoldPartII PDF7 pages
- Piezoelectric Materials: Crystal Orientation and Poling Direction - COMSOL Blog PDFNo ratings yetPiezoelectric Materials: Crystal Orientation and Poling Direction - COMSOL Blog PDF4 pages
- Colloids: Thomas Graham (1861) Studied The Ability of Dissolved Substances ToNo ratings yetColloids: Thomas Graham (1861) Studied The Ability of Dissolved Substances To28 pages
- 2019dec-03 - Ionic Equilibrium - PracticeSheetNo ratings yet2019dec-03 - Ionic Equilibrium - PracticeSheet2 pages
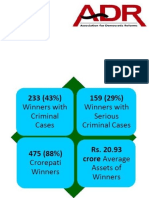
- Analysis Report of Criminal and Financial Background Details of Winners in Lok Sabha 2019 ElectionsNo ratings yetAnalysis Report of Criminal and Financial Background Details of Winners in Lok Sabha 2019 Elections397 pages
- Ionic Equilibrium - Salt Hydrolysis Practice Questions (Level-1)No ratings yetIonic Equilibrium - Salt Hydrolysis Practice Questions (Level-1)3 pages
- Chemistry Organic GOC Reaction Mechanism Elimination E1 E2 E1cB100% (1)Chemistry Organic GOC Reaction Mechanism Elimination E1 E2 E1cB4 pages
- Colligative Properties: Cryoscopy & Ebullios100% (1)Colligative Properties: Cryoscopy & Ebullios30 pages
- Factors Affecting Relative Strengths of Acids and Bases80% (5)Factors Affecting Relative Strengths of Acids and Bases1 page
- The World of Chemistry Episode 5 - A Matter of State0% (1)The World of Chemistry Episode 5 - A Matter of State2 pages
- Diversey Suma Multi d2 3l Cleaner ConcentrateNo ratings yetDiversey Suma Multi d2 3l Cleaner Concentrate2 pages
- Unsupervised Learning of Video Representations Using LstmsNo ratings yetUnsupervised Learning of Video Representations Using Lstms12 pages
- Unless Otherwise Specified, All Monetary Values Are in Millions of INRNo ratings yetUnless Otherwise Specified, All Monetary Values Are in Millions of INR7 pages
- Information and Database Quality Information and Database QualityNo ratings yetInformation and Database Quality Information and Database Quality239 pages
- 22 - AE - 111 - Module - 3 - Recording - Business - TransactionsNo ratings yet22 - AE - 111 - Module - 3 - Recording - Business - Transactions23 pages
- Motivation and Motivational Theory Theories (Industrial Management)100% (8)Motivation and Motivational Theory Theories (Industrial Management)12 pages
- Bank Frauds and Role of RBI - Taxguru - inNo ratings yetBank Frauds and Role of RBI - Taxguru - in3 pages
- Aro Bareilly Recruitment Notification For Agniveer Men 2024-25 Dirzro CC-1707721600No ratings yetAro Bareilly Recruitment Notification For Agniveer Men 2024-25 Dirzro CC-170772160023 pages
- PDF Instruction On Enabling Chinese Font and Typing CharactersNo ratings yetPDF Instruction On Enabling Chinese Font and Typing Characters18 pages
- Essential Oils and Fragrances From NaturNo ratings yetEssential Oils and Fragrances From Natur12 pages