The Karl Fischer Reaction: Stoichiometry
The Karl Fischer Reaction: Stoichiometry
Uploaded by
goparsucoCopyright:
Available Formats
The Karl Fischer Reaction: Stoichiometry
The Karl Fischer Reaction: Stoichiometry
Uploaded by
goparsucoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
The Karl Fischer Reaction: Stoichiometry
The Karl Fischer Reaction: Stoichiometry
Uploaded by
goparsucoCopyright:
Available Formats
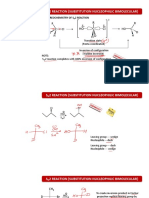
The Karl Fischer reaction
Stoichiometry
(1) CH3OH + SO2 + RN [RNH]SO3CH3
(2) H2O + I2 + [RNH]SO3CH3 + 2 RN [RNH]SO4CH3 + 2 [RNH]I
(RN = Base)
Kinetics
Reaction (1) reaches an equilibrium and produces as reaction intermediate methylsulfite.
Reaction (2) is the oxidation-reduction step. It is very rapid. The rate of the reaction
depends on the base chosen. Pyridine is not suitable for this purpose. Because of its
weak basicity, the methyl sulphurous acid cannot be neutralized completely by pyridine.
The equilibrium (1) is not completely shifted to the right. The reaction is therefore very
slow and the end point is not stable. Because of this lack of stability, the repeatability of
the results is often very poor.
The investigation involved finding amines with a higher basicity and a better affinity for
methyl sulfite. Dr. Scholz chose imidazole. This base shifts the equilibrium (1)
completely to the right. The reaction proceeds at maximum speed and the end point is
very stable. A much greater accuracy can be achieved.
Imidazole seems to be the ideal KF base.
Research at Riedel-de Haën
1979 Beginning of research and development led by Dr. Eugen Scholz
Purpose: To eliminate the unpleasant pyridine as the base.
Results: Pyridine is not a reactant in the KF reaction. lt merely acts as a buffer.
Pyridine can be replaced by bases which are superior for this application.
Action: Determination of bases which optimize the reaction.
Patent application for the use of these bases. Choice of base with the best
buffer capacity: imidazole. Development of new KF reagents using
imidazole as base in the HYDRANAL® reagents.
Developments:
Replacement of halogenated solvents to meet requirements of health care
and environment. Replacement of the toxic methanol with ethanol: new
HYDRANAL®-E Types
New reagents
As result of our research and development KF-Reagents with many advantages are
produced.
high titration speed
stable end points
accurate results
no unpleasant odour
increased safety
Iong storage stability
universal applicability
Regular course of titration
The diagram shows the regular course of the titration of 40 mg water with HYDRANAL®-
Composite using methanol as the working medium. The efficiency of the reagent is
demonstrated by the rapid titration of the free water, as seen from a portion of the curve.
The gradient of this portion of the titration curve is dependent upon the rate of
administration of reagent from the burette. After reaching the 20-second end-point, the
point b (the excess of reagent), shows that the subsequent consumption of the reagent
is virtually zero. This is an indication of the hermeticity of the cell and of the working
technique of the titration.
Vanishing end point
The diagram depicts the titration of 40 mg water in the presence of 5 mL acetone.
Portion b of the curve is no longer vertical and indicates a continual consumption of the
excess reagent and is caused by the ketal formation. The "b" portion of the graph is in
fact a straight line. The rate of this side reaction is indeed constant. The initial water
content of the sample can therefore be evaluated by extrapolation of the b portion of the
curve to t = 0. The origin of the curve, i.e. t = 0, should therefore be carefully deter-
mined. The titration must be started as soon as the sample is added, or, even better,
shortly beforehand (flying start).
The graphical evaluation of the course of a titration enables the real water content of
individual samples to be determined more accurately, in spite of a slow side reaction
taking place. Such a method should always be used when the side reaction cannot be
suppressed by suitable means.
You might also like
- Preparation of 4-Vinylbenzoic Acid by A Wittig Reaction in Aqueous MediumDocument9 pagesPreparation of 4-Vinylbenzoic Acid by A Wittig Reaction in Aqueous MediumohhiNo ratings yet
- Experiment 5 - Double Indicator TitrationDocument16 pagesExperiment 5 - Double Indicator TitrationJoemer Absalon Adorna71% (7)
- Colloidal Silver BookDocument24 pagesColloidal Silver Bookusccc11100% (2)
- Fundamentals of MasstransferandkineticshydrogenationDocument14 pagesFundamentals of MasstransferandkineticshydrogenationRamandhaPrasetyaAdibrataNo ratings yet
- 3 NitrobenzaldehydeDocument4 pages3 Nitrobenzaldehydedave12345No ratings yet
- Quantification of The Maleic Anhydride Grafted Onto Polypropylene by Chemical and Viscosimetric Titrations, and FTIR Spectros PDFDocument11 pagesQuantification of The Maleic Anhydride Grafted Onto Polypropylene by Chemical and Viscosimetric Titrations, and FTIR Spectros PDFThinh DangNo ratings yet
- Synthesis of 5 Cyano IndoleDocument6 pagesSynthesis of 5 Cyano IndolebsureshrajuNo ratings yet
- RDR 6 Quantitative Determination of Oxalate by Permanganate TitrationDocument5 pagesRDR 6 Quantitative Determination of Oxalate by Permanganate TitrationAlyssa Bautista100% (2)
- 541 TitrimetryDocument5 pages541 TitrimetryCristian GomezNo ratings yet
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- ChemDocument2 pagesChemtonsky2002No ratings yet
- Qualitative and Quantitative Analysis1Document18 pagesQualitative and Quantitative Analysis1GHNo ratings yet
- Karl FischerDocument22 pagesKarl FischerManan PatelNo ratings yet
- Water Determination by Karl FischerTitrationDocument81 pagesWater Determination by Karl FischerTitrationtraffik100% (1)
- Test For UnsaturationDocument3 pagesTest For UnsaturationJanaye IfillNo ratings yet
- Karl Fischer Volumetric Titration Theory and Practice: When You Need To Be Sure..Document14 pagesKarl Fischer Volumetric Titration Theory and Practice: When You Need To Be Sure..RENE HERNANDEZNo ratings yet
- Phase Transfer Catalyzed Selective Reduction of Bifunctional MoietiesDocument6 pagesPhase Transfer Catalyzed Selective Reduction of Bifunctional MoietieschemistryjournalNo ratings yet
- Normality N MolarityDocument10 pagesNormality N Molaritynavigcp100% (9)
- Bruttel - Water Determination by Karl Fischer TitrationDocument81 pagesBruttel - Water Determination by Karl Fischer TitrationFernanda LeonNo ratings yet
- Diphenyl AnthraceneDocument3 pagesDiphenyl AnthracenePetr SvobodaNo ratings yet
- Isothermal Batch ReactorDocument10 pagesIsothermal Batch ReactorSaswiny Ritchie0% (2)
- CHE2623 Experiment 1Document7 pagesCHE2623 Experiment 1christellstoltz2No ratings yet
- 1 s2.0 S0926860X98000210 MainDocument11 pages1 s2.0 S0926860X98000210 MainOwen KhosashiNo ratings yet
- Exp Addition Substitution Ver03Document2 pagesExp Addition Substitution Ver03clappedNo ratings yet
- Reverse Phase CHR and PH ControlDocument7 pagesReverse Phase CHR and PH ControlchemistbilimNo ratings yet
- Ajc 33 6 8Document4 pagesAjc 33 6 8IRNSS INDIANo ratings yet
- Quantitative Determination of WaterDocument23 pagesQuantitative Determination of WaterApurba Sarker Apu100% (1)
- The Fischer Esterification of BenzocaineDocument5 pagesThe Fischer Esterification of BenzocaineMikeNo ratings yet
- Karl Fischer - NewDocument4 pagesKarl Fischer - Newfadlysuriansyah100% (1)
- Organic Chemistry Lab ReportDocument12 pagesOrganic Chemistry Lab Reportcyc5326100% (1)
- Fotoderad FlavisDocument4 pagesFotoderad FlavisHylze ChavesNo ratings yet
- 123Document6 pages123Julius Rafael Delprado DildigNo ratings yet
- Acidity of Water: Experiment 5Document11 pagesAcidity of Water: Experiment 5ISAAC ZCAR EBLACAS ASOKNo ratings yet
- Pereaksi GrignardDocument8 pagesPereaksi Grignardisya_nurhidaNo ratings yet
- Ie2020608Document9 pagesIe2020608wiam wiamNo ratings yet
- OrgochemsampleworkDocument10 pagesOrgochemsampleworkMakcaNo ratings yet
- AquametryDocument36 pagesAquametryFoyz Ahmed Shohel80% (5)
- Synthesis of Cu (II) - TetraphenylporphinateDocument7 pagesSynthesis of Cu (II) - Tetraphenylporphinatemiabil100% (1)
- 51LB Week5 7 W13 Background 2 PDFDocument5 pages51LB Week5 7 W13 Background 2 PDFValine Cysteine MethionineNo ratings yet
- Orgo Lab.Document9 pagesOrgo Lab.ladyjacket42No ratings yet
- Hydrogenation of Nitrobenzene To P-Aminophenol in A Four-Phase Reactor Reaction Kinetics and Mass Transfer EffectsDocument6 pagesHydrogenation of Nitrobenzene To P-Aminophenol in A Four-Phase Reactor Reaction Kinetics and Mass Transfer EffectsHeylenLoperaNo ratings yet
- Iodide-Catalyzed Reductions: Development of A Synthesis of Phenylacetic AcidsDocument6 pagesIodide-Catalyzed Reductions: Development of A Synthesis of Phenylacetic AcidsMike Roller100% (1)
- The Gomberg-Bachmann Reaction For The Arylation of Anilines With Aryl DiazotatesDocument5 pagesThe Gomberg-Bachmann Reaction For The Arylation of Anilines With Aryl DiazotatesradhikaNo ratings yet
- Lab 10b Chem 243bDocument3 pagesLab 10b Chem 243bnemesisvirus25No ratings yet
- Aluminium HydroxideDocument5 pagesAluminium HydroxidesnakovaNo ratings yet
- Determination of Fluoride in Water by Reversed-Phase High-Performance Liquid Chromatography Using F-La3+-Alizarin Complexone Ternary ComplexDocument3 pagesDetermination of Fluoride in Water by Reversed-Phase High-Performance Liquid Chromatography Using F-La3+-Alizarin Complexone Ternary ComplexpvsnmalleshNo ratings yet
- Titrimetric Methods of AnalysesDocument10 pagesTitrimetric Methods of AnalysesJason BakerNo ratings yet
- Lab 4Document8 pagesLab 4NelvianaNo ratings yet
- Ionic Liquids Catalyzed Biginelli Reaction Under Solvent-Free ConditionsDocument3 pagesIonic Liquids Catalyzed Biginelli Reaction Under Solvent-Free ConditionsnileshsalunkheNo ratings yet
- Syn Final Helen MDocument8 pagesSyn Final Helen Mapi-509765936No ratings yet
- Kelm 206Document14 pagesKelm 206Soumik MukhopadhyayNo ratings yet
- EX QuestionsDocument5 pagesEX QuestionsJenarthanan Lai Xiong QiNo ratings yet
- Referensi FotofentonDocument5 pagesReferensi FotofentonNurillahi Febria LeswanaNo ratings yet
- Permanganate TitrationDocument6 pagesPermanganate Titrationxavier bourret sicotte83% (6)
- Bromination ExperimentDocument9 pagesBromination Experimentch_ymyaaNo ratings yet
- SOP AMBL 103A AlkalinityDocument5 pagesSOP AMBL 103A AlkalinityFatima AnwarNo ratings yet
- A New Synthesis of (2-'C) Phenobarbital: DiscussionDocument2 pagesA New Synthesis of (2-'C) Phenobarbital: DiscussionkndnsrrfNo ratings yet
- TitrationDocument10 pagesTitrationhao GamesNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeFrom EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeNo ratings yet
- Typical Tests Carried Out On LubricantsDocument34 pagesTypical Tests Carried Out On Lubricantsgoparsuco100% (1)
- KFD2 RR Ex1Document4 pagesKFD2 RR Ex1goparsucoNo ratings yet
- KFD2 CD2 Ex1Document3 pagesKFD2 CD2 Ex1goparsucoNo ratings yet
- KFD2 SR2 Ex2.WDocument4 pagesKFD2 SR2 Ex2.WgoparsucoNo ratings yet
- Miniature Temperature Sensors: For Miniature Bearing and Babbitt Bearing ApplicationsDocument4 pagesMiniature Temperature Sensors: For Miniature Bearing and Babbitt Bearing ApplicationsgoparsucoNo ratings yet
- Globe Valve Type Flow Switch: DescriptionDocument4 pagesGlobe Valve Type Flow Switch: DescriptiongoparsucoNo ratings yet
- Medical BiochemistryDocument264 pagesMedical BiochemistryKarren Taquiqui Plete100% (1)
- Chem 114 SyllabusDocument8 pagesChem 114 SyllabusCheska BiolenaNo ratings yet
- SelectfellowsDocument26 pagesSelectfellowsSougata HalderNo ratings yet
- Mathematics 1st YearDocument13 pagesMathematics 1st Yearnolting raoNo ratings yet
- TABIANAN Major Requirement - 2 Curriculum Plan - PDF - PDFDocument6 pagesTABIANAN Major Requirement - 2 Curriculum Plan - PDF - PDFJeramy BallesterosNo ratings yet
- Chem MC Content (1) MergedDocument171 pagesChem MC Content (1) MergedjlkdinhkNo ratings yet
- 343706Document235 pages343706Raluca Iuliana ButoiNo ratings yet
- Organic Synthesis WorksheetDocument2 pagesOrganic Synthesis WorksheetJimmy YeNo ratings yet
- Chapter10 1 (Alkene)Document65 pagesChapter10 1 (Alkene)Samina AliNo ratings yet
- REVISED SR SEC Chemistry 2020 21Document8 pagesREVISED SR SEC Chemistry 2020 21jacobNo ratings yet
- (Advances in Inorganic Chemistry and Radiochemistry 21) H.J. Emeléus and A.G. Sharpe (Eds.) - Elsevier, Academic Press (1978)Document315 pages(Advances in Inorganic Chemistry and Radiochemistry 21) H.J. Emeléus and A.G. Sharpe (Eds.) - Elsevier, Academic Press (1978)Thảo HàNo ratings yet
- Heuristics For Process SynthesisDocument26 pagesHeuristics For Process SynthesisThien LeNo ratings yet
- IISER BSMS Curriculum 2021Document162 pagesIISER BSMS Curriculum 2021ADWYTH GNAIRNo ratings yet
- Lesson 12 Chemical IncompatibilitiesDocument16 pagesLesson 12 Chemical IncompatibilitiesAngelica GomezNo ratings yet
- BTech AEIE Syllabus June2019Document80 pagesBTech AEIE Syllabus June2019Buzz PriyanshuNo ratings yet
- Revised GCSE CHEM REVISED Support 21916Document7 pagesRevised GCSE CHEM REVISED Support 21916phoebe wongNo ratings yet
- 2020 DSE CHEM 1A Mock Exam PDFDocument13 pages2020 DSE CHEM 1A Mock Exam PDFLai LeonNo ratings yet
- Production of Hydrogen Gas by Steam Reforming ProcessDocument38 pagesProduction of Hydrogen Gas by Steam Reforming ProcessAinggararuban GaneshanNo ratings yet
- Lid /penutup Crucible Magnesium RibbonDocument6 pagesLid /penutup Crucible Magnesium RibbonEIJA_HAFIZA867602No ratings yet
- S 2 Reaction (Substitution Nucleophilic Bimolecular)Document15 pagesS 2 Reaction (Substitution Nucleophilic Bimolecular)Palash ChawhanNo ratings yet
- Pearson Ch2 Answers-1Document12 pagesPearson Ch2 Answers-1Person GainableNo ratings yet
- Physical and Chemical Change Virtual LabDocument2 pagesPhysical and Chemical Change Virtual LabAngel SolivanNo ratings yet
- ICSE MCQ QuestionBankDocument11 pagesICSE MCQ QuestionBankPratapSinghMunia100% (1)
- Electro-Organic SynthesisDocument19 pagesElectro-Organic Synthesisirine100% (1)
- Physical Sciences P2 Grade 11 Exemplar 2013 EngDocument15 pagesPhysical Sciences P2 Grade 11 Exemplar 2013 Engtapiwanashe018No ratings yet
- Urea-Formaldehyde Resins Production ApplicationDocument11 pagesUrea-Formaldehyde Resins Production ApplicationAhid RahmatNo ratings yet
- Enzyme ImmobilizationDocument67 pagesEnzyme ImmobilizationBijayaKumarUpretyNo ratings yet
- MCQ TestDocument5 pagesMCQ TestPrajwal KaleNo ratings yet
- DFT Study Magnesium Corrosion PDFDocument11 pagesDFT Study Magnesium Corrosion PDFVerica VulovicNo ratings yet