Thermodynamics 101 - 1
Thermodynamics 101 - 1
Uploaded by
Joseph DustCopyright:
Available Formats
Thermodynamics 101 - 1
Thermodynamics 101 - 1
Uploaded by
Joseph DustOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Thermodynamics 101 - 1
Thermodynamics 101 - 1
Uploaded by
Joseph DustCopyright:
Available Formats
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and
their relation to energy, entropy, and the physical properties of matter and radiation. The
behavior of these quantities is governed by the four laws of thermodynamics which
convey a quantitative description using measurable macroscopic physical quantities,
but may be explained in terms of microscopic constituents by statistical mechanics.
Thermodynamics applies to a wide variety of topics in science and engineering,
especially physical chemistry, biochemistry, chemical engineering and mechanical
engineering, but also in other complex fields such as meteorology.
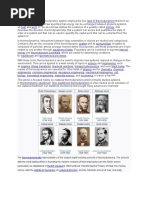
Historically, thermodynamics developed out of a desire to increase the efficiency of early
steam engines, particularly through the work of French physicist Sadi Carnot (1824) who
believed that engine efficiency was the key that could help France win the Napoleonic
Wars.[1] Scots-Irish physicist Lord Kelvin was the first to formulate a concise definition
of thermodynamics in 1854[2] which stated, "Thermo-dynamics is the subject of the
relation of heat to forces acting between contiguous parts of bodies, and the relation of
heat to electrical agency." German physicist and mathematician Rudolf Clausius
restated Carnot's principle known as the Carnot cycle and gave to the theory of heat a
truer and sounder basis. His most important paper, "On the Moving Force of Heat",[3]
published in 1850, first stated the second law of thermodynamics. In 1865 he introduced
the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat.[4]
The initial application of thermodynamics to mechanical heat engines was quickly
extended to the study of chemical compounds and chemical reactions. Chemical
thermodynamics studies the nature of the role of entropy in the process of chemical
reactions and has provided the bulk of expansion and knowledge of the field. Other
formulations of thermodynamics emerged. Statistical thermodynamics, or statistical
mechanics, concerns itself with statistical predictions of the collective motion of
particles from their microscopic behavior. In 1909, Constantin Carathéodory presented
a purely mathematical approach in an axiomatic formulation, a description often referred
to as geometrical thermodynamics.
Introduction
A description of any thermodynamic system employs the four laws of thermodynamics
that form an axiomatic basis. The first law specifies that energy can be transferred
between physical systems as heat, as work, and with transfer of matter.[5] The second
law defines the existence of a quantity called entropy, that describes the direction,
thermodynamically, that a system can evolve and quantifies the state of order of a system
and that can be used to quantify the useful work that can be extracted from the system.[6]
In thermodynamics, interactions between large ensembles of objects are studied and
categorized. Central to this are the concepts of the thermodynamic system and its
surroundings. A system is composed of particles, whose average motions define its
properties, and those properties are in turn related to one another through equations of
state. Properties can be combined to express internal energy and thermodynamic
potentials, which are useful for determining conditions for equilibrium and spontaneous
processes.
With these tools, thermodynamics can be used to describe how systems respond to
changes in their environment. This can be applied to a wide variety of topics in science
and engineering, such as engines, phase transitions, chemical reactions, transport
phenomena, and even black holes. The results of thermodynamics are essential for other
fields of physics and for chemistry, chemical engineering, corrosion engineering,
aerospace engineering, mechanical engineering, cell biology, biomedical engineering,
materials science, and economics, to name a few.[7][8]
This article is focused mainly on classical thermodynamics which primarily studies
systems in thermodynamic equilibrium. Non-equilibrium thermodynamics is often
treated as an extension of the classical treatment, but statistical mechanics has brought
many advances to that field.
You might also like
- (Student's Guides) Lemons, D.S. - A Student's Guide To Dimensional Analysis-Cambridge University Press (2017) PDFDocument115 pages(Student's Guides) Lemons, D.S. - A Student's Guide To Dimensional Analysis-Cambridge University Press (2017) PDFAnonymous 24lnhh100% (1)
- RatioDocument34 pagesRatioNorliza SapatanohNo ratings yet
- ThermdynamicsDocument2 pagesThermdynamicspirqayum1No ratings yet
- ThermodynamicsDocument1 pageThermodynamicsThilaksiri BandaraNo ratings yet
- Thermodynamics: Jump To Navigation Jump To SearchDocument16 pagesThermodynamics: Jump To Navigation Jump To SearchAkarshan WaliaNo ratings yet
- Thermodynamics: Jump To Navigation Jump To SearchDocument16 pagesThermodynamics: Jump To Navigation Jump To SearchAkarshan WaliaNo ratings yet
- A Guide To Get A Top 50 Rank in JEEDocument1 pageA Guide To Get A Top 50 Rank in JEEAdarsh50% (2)
- ThermodynamicsDocument14 pagesThermodynamicsEurich EstradaNo ratings yet
- Thermodynamics Is The Science Of: o o o o oDocument15 pagesThermodynamics Is The Science Of: o o o o oPratik YadavNo ratings yet
- Thermodynamics: Thermodynamics Is A Branch of Physics Concerned With Heat andDocument10 pagesThermodynamics: Thermodynamics Is A Branch of Physics Concerned With Heat andJayaNo ratings yet
- ThermdynamcsDocument13 pagesThermdynamcsTejas krishnakanthNo ratings yet
- ThermodynamicsDocument45 pagesThermodynamicsJaquesNo ratings yet
- ThermoDocument1 pageThermoJuan RosalesNo ratings yet
- Team ThermalDocument11 pagesTeam ThermalDamisha DamishaNo ratings yet
- ThermodynamicsDocument292 pagesThermodynamicsxitta0092% (12)
- Thermodynamics: Thermodynamics Is A Branch ofDocument22 pagesThermodynamics: Thermodynamics Is A Branch ofPradeep KumarNo ratings yet
- Thermodynamics - WikipediaDocument78 pagesThermodynamics - WikipediasubhajitparichhaNo ratings yet
- Hermodynamics Is The Branch Of: System SurroundingsDocument8 pagesHermodynamics Is The Branch Of: System SurroundingsDamisha DamishaNo ratings yet
- Thermodynamic SystemDocument9 pagesThermodynamic SystemJordan MosesNo ratings yet
- Thermodynamics Is A Branch Of: (Hide)Document5 pagesThermodynamics Is A Branch Of: (Hide)ksrm123No ratings yet
- Thermodynamics Is The Branch ofDocument2 pagesThermodynamics Is The Branch ofbennyNo ratings yet
- IndexDocument286 pagesIndexTuan HoangNo ratings yet
- Thermodynamics - WikipediaDocument15 pagesThermodynamics - Wikipediapsri1944No ratings yet
- Thermodynamics: From Wikipedia, The Free EncyclopediaDocument8 pagesThermodynamics: From Wikipedia, The Free Encyclopediaeaglebaby11No ratings yet
- ThermodynamicsDocument14 pagesThermodynamicsdanny222No ratings yet
- Thermodynamics: From Wikipedia, The Free EncyclopediaDocument26 pagesThermodynamics: From Wikipedia, The Free Encyclopediaglh00No ratings yet
- ThermodynamicsDocument15 pagesThermodynamicspsri1944No ratings yet
- Parts of Bodies, and The Relation of Heat To Electrical Agency."Document1 pageParts of Bodies, and The Relation of Heat To Electrical Agency."JejeNo ratings yet
- Thermodynamics Is The Branch ofDocument1 pageThermodynamics Is The Branch ofSaad AhsanNo ratings yet
- Thermodynamics - WikipediaDocument15 pagesThermodynamics - Wikipediapsri1944No ratings yet
- Thermodynamics Is A Branch ofDocument1 pageThermodynamics Is A Branch ofraghumavathurNo ratings yet
- Thermodynamics Is A Branch ofDocument1 pageThermodynamics Is A Branch ofGTNo ratings yet
- Ae31001 Thermodynamics and Aerospace Propulsion System: Subject Teachers - PROF. S. Karmakar/Prof. R. JoarderDocument4 pagesAe31001 Thermodynamics and Aerospace Propulsion System: Subject Teachers - PROF. S. Karmakar/Prof. R. JoarderAranya DanNo ratings yet
- ThermodynamicsDocument10 pagesThermodynamicsMartin Ignacio Mendieta LoraNo ratings yet
- MEFC0303-Report 01Document13 pagesMEFC0303-Report 01Jermain Liam EscuderoNo ratings yet
- Thermodynamics NotesDocument2 pagesThermodynamics Notespetidal262No ratings yet
- ThermodynamicsDocument2 pagesThermodynamicsIlyass ChahoudNo ratings yet
- Thermodynamics:: Thermodynamics Is The Science ofDocument3 pagesThermodynamics:: Thermodynamics Is The Science ofSuleman SaleemNo ratings yet
- ThermodynamicsDocument1 pageThermodynamicsKevin Walf H. AñonuevoNo ratings yet
- Dynamis, Meaning "Document8 pagesDynamis, Meaning "Chithra G DasNo ratings yet
- Thermodynamics: A Dynamical Systems ApproachFrom EverandThermodynamics: A Dynamical Systems ApproachRating: 5 out of 5 stars5/5 (4)
- Thermodynamics SummaryDocument3 pagesThermodynamics Summarykiyasi5148No ratings yet
- Thermo Handouts 20110Document36 pagesThermo Handouts 20110jonnydp50No ratings yet
- Entropy: Navigation SearchDocument18 pagesEntropy: Navigation SearchAbhishek HoroNo ratings yet
- Chemistry Notes .. Energetic .. O Levels ..: EnergeticsDocument5 pagesChemistry Notes .. Energetic .. O Levels ..: Energeticsaceer ...100% (2)
- Assignment On ThermodynamicsDocument4 pagesAssignment On ThermodynamicsmickyNo ratings yet
- Laws of ThermodynamicsDocument6 pagesLaws of ThermodynamicszzaanNo ratings yet
- History of ThermodynamicsDocument10 pagesHistory of ThermodynamicsTasya Fara99No ratings yet
- 1310 0241v1 PDFDocument341 pages1310 0241v1 PDFMefNo ratings yet
- ENSC 14A - Chapter 1Document40 pagesENSC 14A - Chapter 1deusleanNo ratings yet
- Thermodynamics: Jump To Navigationjump To SearchDocument4 pagesThermodynamics: Jump To Navigationjump To SearchLawrence DecanoNo ratings yet
- Physical ChemistryDocument28 pagesPhysical ChemistryGail Jonah AstorgaNo ratings yet
- Thermo. 1-13Document13 pagesThermo. 1-134501-Abdullah MasoodNo ratings yet
- Module 1: Classical Thermodynamics Lecture 1: Review of ThermodynamicsDocument2 pagesModule 1: Classical Thermodynamics Lecture 1: Review of ThermodynamicsDr. M. Mohan Jagadeesh kumarNo ratings yet
- Introduction, History and Applications: Engr. Shakeel AhmadDocument13 pagesIntroduction, History and Applications: Engr. Shakeel AhmadShakeel MohmandNo ratings yet
- Thermodynamics Kalanov13Document9 pagesThermodynamics Kalanov13Zuhaa FachraniNo ratings yet
- Thermodynamics Lecture 1Document30 pagesThermodynamics Lecture 1Ssegirinya WilberforceNo ratings yet
- Cangel & Boles TranslateDocument16 pagesCangel & Boles TranslateRidho Dwi SyahrialNo ratings yet
- Chapter 1 - Introduction ThemoDocument43 pagesChapter 1 - Introduction ThemoOctGolibib100% (1)
- What Is Thermodynamics and Write It's Application..Document2 pagesWhat Is Thermodynamics and Write It's Application..Prashant Kumar MahantaNo ratings yet
- Laws of ThermodynamicsDocument16 pagesLaws of ThermodynamicsRODULFO V. PAQUINGANNo ratings yet
- The Thermodynamic Universe and Beyond: How Nature's Laws Reveal the Secrets of Time, Biology, Information, and Quantum RealityFrom EverandThe Thermodynamic Universe and Beyond: How Nature's Laws Reveal the Secrets of Time, Biology, Information, and Quantum RealityNo ratings yet
- Least Mastered Competencies in MATH Palingowak ESDocument4 pagesLeast Mastered Competencies in MATH Palingowak ESMay Anne Almario100% (4)
- Approximate Bayesian Computation (ABC) in PracticeDocument9 pagesApproximate Bayesian Computation (ABC) in PracticeJanardan DasNo ratings yet
- MaTh 7.5 ExamplesDocument2 pagesMaTh 7.5 ExamplesCA-Math-TeachNo ratings yet
- Statistics Miscellaneous QuestionsDocument3 pagesStatistics Miscellaneous QuestionsNipun GoyalNo ratings yet
- Generic: Process Step Illustration 1.3Document4 pagesGeneric: Process Step Illustration 1.3idenbelleNo ratings yet
- Exclusive Topics: Class 1 To Class 12 Circle Geometry Class VI To X Industry Sector AnalysisDocument4 pagesExclusive Topics: Class 1 To Class 12 Circle Geometry Class VI To X Industry Sector AnalysisamitjustamitNo ratings yet
- Homogeneous Differential EquationDocument8 pagesHomogeneous Differential EquationSyed TuhinNo ratings yet
- Assignment Pelajar Add Math f4Document6 pagesAssignment Pelajar Add Math f4Zulhanif IdrisNo ratings yet
- UMN BS Electrical EngineeringDocument6 pagesUMN BS Electrical EngineeringRyan LockeNo ratings yet
- Wavelet Based WatermarkingDocument43 pagesWavelet Based Watermarkingdj0808No ratings yet
- ABE 314 NOTE (Autosaved) - 2Document62 pagesABE 314 NOTE (Autosaved) - 2dayo JohnsonNo ratings yet
- Applications of Calculus in Civil EngineeringDocument10 pagesApplications of Calculus in Civil EngineeringEllitheusNo ratings yet
- Course Outline Linear AlgebraDocument2 pagesCourse Outline Linear AlgebraZahid IbkNo ratings yet
- Information Sciences: Li Kong, Chuanyi Li, Jidong Ge, Feifei Zhang, Yi Feng, Zhongjin Li, Bin LuoDocument17 pagesInformation Sciences: Li Kong, Chuanyi Li, Jidong Ge, Feifei Zhang, Yi Feng, Zhongjin Li, Bin LuoSabbir HossainNo ratings yet
- KD Kakuro 5x5 v3 s2 b065Document3 pagesKD Kakuro 5x5 v3 s2 b065RADU GABINo ratings yet
- Box Operator+ (Const Box&)Document2 pagesBox Operator+ (Const Box&)Vivekanandhan VijayanNo ratings yet
- Imo Sample Paper Class-6Document2 pagesImo Sample Paper Class-6Anirudh PadegalNo ratings yet
- Writing Good PI ObjectivesDocument42 pagesWriting Good PI Objectivesfrankiepaul06No ratings yet
- Careers in Civil EngineeringDocument27 pagesCareers in Civil Engineeringbadrul79No ratings yet
- ConjugateDocument7 pagesConjugateAnonymous 4oyclVHtAmNo ratings yet
- Study of Combinational and Booth Multiplier: Neha Goyal, Khushboo Gupta, Renu SinglaDocument4 pagesStudy of Combinational and Booth Multiplier: Neha Goyal, Khushboo Gupta, Renu SinglajdNo ratings yet
- Sample Paper 11Document18 pagesSample Paper 11twisha.guptaNo ratings yet
- Kyc Maths Class 12 P4 2023-24Document6 pagesKyc Maths Class 12 P4 2023-24soumityachaudharyNo ratings yet
- Syllabus BS 4years Economics PDFDocument114 pagesSyllabus BS 4years Economics PDFJaveria WahabNo ratings yet
- Rithmetic and Eometric Rogression: Topical Worksheet: Arithmetic and Geometric ProgressionsDocument2 pagesRithmetic and Eometric Rogression: Topical Worksheet: Arithmetic and Geometric ProgressionsTim Gan MathNo ratings yet
- TNPSC Aptitude Solved Sums Part 1Document13 pagesTNPSC Aptitude Solved Sums Part 1VickyNo ratings yet
- Bi-Directional Safety GearsDocument9 pagesBi-Directional Safety GearsFERNSNo ratings yet
- POGIL - MeasurementDocument5 pagesPOGIL - MeasurementPhillip CookNo ratings yet