Air Leakage in Vacuum Vessels
Air Leakage in Vacuum Vessels
Uploaded by
Accio_thebestCopyright:
Available Formats
Air Leakage in Vacuum Vessels
Air Leakage in Vacuum Vessels
Uploaded by
Accio_thebestCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Air Leakage in Vacuum Vessels
Air Leakage in Vacuum Vessels
Uploaded by
Accio_thebestCopyright:
Available Formats
Air Leakage in Vacuum Vessels
The chart, Fig. 1, is calculated from the formula: . 0.071*. p V ML = 0,071 * A t Where: MA = Air leakage in kg/h
p = Change of pressure in mbar p
t V
= Corresponding time in min Air leakage in kg/hr = Plant volume in m
How airtight is a vacuum plant? Is the suction capacity of the vacuum pump large enough? Why does it take so long for the plant to reach the vacuum? Must the vacuum pump extract leak air as well as gases from the product? Evacuate the vessel to a vacuum under 500 mbar, e. g. 60 mbar. Isolate the vacuum pump from the vessel and completely seal off the vessel. Measure the pressure increase in the vessel and determine the corresponding time. The pressure increase in mbar divided by the time in minutes gives the vacuum loss in mbar/minute. With this value and the volume of the vessel under vacuum the air leakage rate in kg/hr can be found in chart fig.1.
Vacuum loss in mbar/min
V= Plant volume
Fig 1
* the exact value is 0.071289977 based on: J universal gas constant = 8.31441 ,
mol K
absolute Temperature 293.15 K and mol mass for air 28.96 kg/kmol
Example: A vessel of 20 m volume is evacuated to 60 mbar and isolated. Within 10 minutes the vacuum drops to 120 mbar = p 60 mbar. The pressure change is 60 : 10 = 6 mbar/minute, therefore the vacuum loss is 6 mbar/minute. From the chart fig. 1 an air leakage rate of 8.5 kg/h is found.
In the high vacuum range the air leakage rate or the quantities of gases and vapours are measured in mbar litre/s. 1 mbar litre/s 0.0043 kg/hr air at 20 C
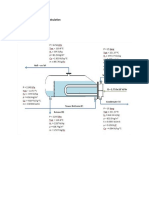
Admissible Velocity of Flow in Vacuum Pipelines
The admissible velocity of flow in a vacuum pipeline depends on how high the pressure loss of this pipeline is allowed to be. Higher pressure loss means increased energy requirements for the vacuum pump. A pressure loss of 10 % of the total pressure can generally be accepted. This is shown in graph fig. 1 and valid for air at 20 C. It is calculated according to the formula: wzul Wadm = wadm R 0.2 R T 0,2 . R . T . l 1 + 40 d = admissible velocity of flow in m/s = gas constant in J/kg K
R= r m
For reasons of simplication, the calculation is based on an average pipe friction coefficient of = 0.04 (this is ~ max.) and on a free-of-loss acceleration from 0 to w m/s, i. e. with a well rounded pipeline inlet. Range: 2 mbar p 1000 mbar. Furthermore the graph contains lines for constant volume flow V in m/hr. The graph is meant for rapid, rough dimensioning of a vacuum line. An exact pressure loss calculation can be made with the help of sheet 2000 abl 9.
r = 8314.3 J/kmol K= universal gas constant m = Mol mass in kg/kmol T I d = Temperature in K = Length of pipeline in m = Diameter of pipeline in mm
A 15
2000 abl 8
Admissible Velocity of Flow in Vacuum Pipelines
Admissible velocity of flow in m/s
p at p = 0.1 or 10%
Nominal diameter of pipeline in mm Fig 2
l= lenght of pipeline, V= volume flow
Example: calculation of mass flow Given: Pipeline ND 100 Length of pipeline I = 10 m Flow medium: Air at 20 C Total pressure p = 10 mbar Wanted: 1) Admissible velocity wadm in m/s 2) Volume flow V in m/hr 3) Mass flow M in kg/hr From the graph fig. 1 can be found: 1) wadm u 58m/s 2) V u 1650 m/hr Calculation: 3)
M= V kg/hr kg / h v
840 3 m / kg p vA = specific volume of air at 20C
vA = vL
If in the above example values for water vapour with a temperature = 7 C, p = 10 mbar and m = 18 kg/kmol are inserted, the following formula results: wadm = f wadm =
273 + 7 . 58 72 m / s . 18 10
p = pressure in mbar: . 1650 10 kg 20kg M= 840 Conversion to other gases and vapours If instead of air at 20 C another gas or vapour at the temperature in C is to be conveyed, the values from the graph have to be multiplied by the factor:
f= 273 + . m 10
from diagram fig. 2: Volume flow V u 2050 m/hr by calculation: Mass flow
M' = V' kg/hr kg / h v'
m = Mol mass of gas or vapours
from Water vapour temperature table zu = 7C; v = 129.1 m/kg therefore: 2050 M' = 16 kg / h kg/hr 129.1 129,1
Equivalent length of pipeline of pipe bends and gate valves are installed in the pipeline
I = I + e d . 40
Example Given are: Pipeline DN 600 Length of pipeline l = 100 m Flowing medium: air at 20C Total pressure p = 10 mbar 4 pipe bends 90; 1 gate valve Wanted: 1) Equivalent length of pipeline Ie in m 2) Admissible velocity of flow wadm in m/s 3) Volume flow V in m/hr 4) Mass flow in M kg/hr
By calculation we find: 1) 600 . . Ie = 100 + (4 0,16 + 1) 40 = 124.6 m From graph fig. 2 we find: 2) wadm u 44 m/s 3) V Hence: 4)
M=
Ie I d
= equivalent length of pipeline in m = length of pipeline in m = diameter of pipeline in mm = resistance coefficients: pipe bend D/d = 3; 90 = 0.16 gate valve with restriction = 1.0
u 45000 m/hr
45000 . 10 u kg / h kg/hr 535 535 840
A 16
You might also like
- Steam Ejector CalculationsDocument9 pagesSteam Ejector CalculationsN. S. Pandit100% (6)
- Steam Ejector CalculationsDocument11 pagesSteam Ejector Calculationsrashm006ranjan100% (3)
- Jacket Area CalculationDocument12 pagesJacket Area CalculationPraveen Prajapati100% (2)
- Thermo Compressor CalculatorDocument6 pagesThermo Compressor CalculatorVilas m ChinkeNo ratings yet
- Line Sizing ReportDocument7 pagesLine Sizing Reportmichael chiwuezeNo ratings yet
- ReboilerDocument4 pagesReboilerKim Lloyd A. Barrientos100% (1)
- Line Sizing For Two Phase FlowDocument3 pagesLine Sizing For Two Phase FlowJaydeep Patel100% (1)
- Vacum Calculation ExampleDocument18 pagesVacum Calculation ExampleAgung SiswahyuNo ratings yet
- 325 - Sparger DesignDocument3 pages325 - Sparger DesignInggit Prillasari100% (1)
- Centrifugal Pumps: Process Calculation / Data SheetDocument3 pagesCentrifugal Pumps: Process Calculation / Data SheetMurali MuthuNo ratings yet
- Ammonia Wet Scrubber System (17-7-2018)Document12 pagesAmmonia Wet Scrubber System (17-7-2018)addin wokatuba50% (2)
- Mechanical Vapor RecompressionDocument9 pagesMechanical Vapor Recompressionnarayana reddy0% (1)
- PSV Sizing Tool API Based Calc Sheets PDFDocument11 pagesPSV Sizing Tool API Based Calc Sheets PDFLucas Farias0% (1)
- Heat Transfer Design Methods by John MC Ketta PDFDocument607 pagesHeat Transfer Design Methods by John MC Ketta PDFAlberto LazzarettiNo ratings yet
- Molecular Sieve Sizing Sheet PematangDocument8 pagesMolecular Sieve Sizing Sheet Pematangahmad santoso100% (1)
- LP HP Dosing CalculationDocument1 pageLP HP Dosing Calculationvikas_be1750% (4)
- Insulation ThicknessDocument2 pagesInsulation ThicknessShashi Kant KumarNo ratings yet
- Line Sizing GuidelinesDocument31 pagesLine Sizing Guidelinesc_nghia100% (3)
- Vacuum Catch PotDocument1 pageVacuum Catch Potsushant_jhawerNo ratings yet
- Estimating Air LeakageDocument5 pagesEstimating Air Leakagenasirmuzaffar100% (1)
- Vapor Line Sizing-Mpp6Document10 pagesVapor Line Sizing-Mpp6Nitin KurupNo ratings yet
- Calculation Pressure DropDocument9 pagesCalculation Pressure Dropdasubhai0% (1)
- (HOW TO) Select Required Vacuum Pump Capacity - Pharma EngineeringDocument11 pages(HOW TO) Select Required Vacuum Pump Capacity - Pharma EngineeringpratikNo ratings yet
- Boiling Up Rate For Batch DistillationDocument4 pagesBoiling Up Rate For Batch DistillationKanhaiyalal PatilNo ratings yet
- Steam Cost Calculations 2020Document3 pagesSteam Cost Calculations 2020Malwadkar P.B.No ratings yet
- Restriction Orifice Sizing Calculation: P (kg/ms2)Document2 pagesRestriction Orifice Sizing Calculation: P (kg/ms2)SiLan Subramaniam100% (1)
- Design Conditions CriteriaDocument4 pagesDesign Conditions CriteriaJaldhi Patel100% (2)
- Limpet Coil Reactor - 28-05Document3 pagesLimpet Coil Reactor - 28-05AJAY138150% (2)
- Limpet Coil CalculationDocument1 pageLimpet Coil Calculationkirandas_mullassery100% (1)
- How ToDocument45 pagesHow ToDHAVALESH AMNo ratings yet
- Rupture Disk SizingDocument2 pagesRupture Disk Sizingabhishek shindeNo ratings yet
- Steam Ejector DesignDocument4 pagesSteam Ejector DesignHaider Tyebally TyeballyNo ratings yet
- Calculation of Ejector Jet Engine: Thrust 119.8 NDocument2 pagesCalculation of Ejector Jet Engine: Thrust 119.8 Njbrferracin782No ratings yet
- Handbook of Chemical Engineering Calculations, Third Edition. Nicholas P. ChopeyDocument3 pagesHandbook of Chemical Engineering Calculations, Third Edition. Nicholas P. ChopeyRay RichalmyNo ratings yet
- Reboilers PDFDocument0 pagesReboilers PDFtpchoNo ratings yet
- Sizing of Steam Coil of Insulated Tank Rev 3 PDFDocument2 pagesSizing of Steam Coil of Insulated Tank Rev 3 PDFdhavalesh1No ratings yet
- Mist Eliminator DesignDocument2 pagesMist Eliminator DesignEng Alf75% (4)
- Limpet Coil Area CalculationDocument6 pagesLimpet Coil Area CalculationPraveen Prajapati100% (4)
- Heat Loss CalculationDocument12 pagesHeat Loss CalculationRajaramNo ratings yet
- Condenser Design CalculationDocument11 pagesCondenser Design CalculationAlMakhie0% (1)
- Design of Distillation Columns - ImpDocument13 pagesDesign of Distillation Columns - ImpAlla VijayNo ratings yet
- Distillation Column Design CalculationDocument6 pagesDistillation Column Design Calculationmynurul100% (1)
- Steam Ejector HysysDocument4 pagesSteam Ejector Hysysfluto100% (1)
- AgitatorDocument6 pagesAgitatorSung Woong MoonNo ratings yet
- Design & Performance Evaluation of Steam EjectorDocument6 pagesDesign & Performance Evaluation of Steam EjectorAnonymous UY9QffNo ratings yet
- PSV Scenario and CalculationDocument35 pagesPSV Scenario and CalculationBaba Johneh67% (3)
- Thermosyphon Reboiler HydraulicsDocument2 pagesThermosyphon Reboiler HydraulicsMichael HaiseNo ratings yet
- Blowdown Orifice Sizing CalculationDocument1 pageBlowdown Orifice Sizing Calculationlutfi awnNo ratings yet
- ReactiveDistillation ReviewDocument17 pagesReactiveDistillation ReviewSirajuddinNo ratings yet
- Cepde - Valve Sizing (U16ch036, U16ch037, U16ch083)Document15 pagesCepde - Valve Sizing (U16ch036, U16ch037, U16ch083)VRUSHABHNo ratings yet
- Imtp High-Performance Random Packing: Norton Chemical Process Products CorporationDocument16 pagesImtp High-Performance Random Packing: Norton Chemical Process Products Corporationchemkumar16100% (1)
- GEA Vacuum Line Sizing & LossesDocument2 pagesGEA Vacuum Line Sizing & Lossesrajindo1100% (1)
- Assignment Sheet No 2 - 2016Document8 pagesAssignment Sheet No 2 - 2016Muhammad Haseeb JavedNo ratings yet
- Tutorial 3 - BMCF 2223 (Manometer)Document4 pagesTutorial 3 - BMCF 2223 (Manometer)Muhamad Syazwan100% (1)
- Mebc Tutorial 01Document3 pagesMebc Tutorial 01Deep KhuntNo ratings yet
- Che 222 Tutorial 2Document15 pagesChe 222 Tutorial 2naverfallNo ratings yet
- Mebc CH 1 Questions of GtuDocument3 pagesMebc CH 1 Questions of GtuDeep Khunt100% (1)
- Kailash WT Perf AnalysisDocument20 pagesKailash WT Perf AnalysisNahas NazarNo ratings yet
- S06-All Examples 2upDocument5 pagesS06-All Examples 2upAvinash MeenaNo ratings yet
- Absorption NotesDocument79 pagesAbsorption Noteshanisshi100% (2)
- Air-Conditioning Lexicon - A To Z Index - StulzDocument7 pagesAir-Conditioning Lexicon - A To Z Index - StulzAnkesh RajNo ratings yet
- KNS 1073 Assignment 1 Muhammad KhairinDocument6 pagesKNS 1073 Assignment 1 Muhammad KhairinMuhammad khairinNo ratings yet
- BrochureDocument16 pagesBrochureDaniel A. NorbergNo ratings yet
- Raft FoundationDocument7 pagesRaft FoundationSouliman AlkhoderNo ratings yet
- Pips Technical Service Price ListDocument5 pagesPips Technical Service Price ListAsyraf RefdinNo ratings yet
- Heat and Mass Transfer1Document173 pagesHeat and Mass Transfer1Subramanian ChidambaramNo ratings yet
- Practical Nonlinear Dynamic Analysis of Cable Stayed BridgesDocument4 pagesPractical Nonlinear Dynamic Analysis of Cable Stayed Bridgesdhanush100% (2)
- Vertical Thrust Block Design (DN700&DN 800)Document28 pagesVertical Thrust Block Design (DN700&DN 800)ARSENo ratings yet
- Final Neet (Ug) - 2023 Phase 2 (Manipur Examination) Test Paper With Answer & Detailed Solution (Held On Tuesday 6th JUNE, 2023)Document37 pagesFinal Neet (Ug) - 2023 Phase 2 (Manipur Examination) Test Paper With Answer & Detailed Solution (Held On Tuesday 6th JUNE, 2023)aiimstarget2024No ratings yet
- 16-PT5 Durst Pump DrivesDocument5 pages16-PT5 Durst Pump DrivesDiego AlbarracinNo ratings yet
- Study & Analysis of Transportation SkidDocument10 pagesStudy & Analysis of Transportation SkidJay PNo ratings yet
- CR ArtifactsDocument69 pagesCR ArtifactsPriyanka Modi100% (2)
- Advantages of Code Case 2695 and Comparison Bet ASME Div 1 and Div 2 PDFDocument42 pagesAdvantages of Code Case 2695 and Comparison Bet ASME Div 1 and Div 2 PDFPrashant Agrawal100% (1)
- Coolant Circuit bw212Document2 pagesCoolant Circuit bw212Gabriel Paredes E.No ratings yet
- Chemistry 1Document58 pagesChemistry 1Ash VinNo ratings yet
- Strong and Weak AcidsDocument22 pagesStrong and Weak AcidsRohini SelvarajahNo ratings yet
- Two-Level SystemDocument6 pagesTwo-Level System蔡蓁羚No ratings yet
- Chapter 2Document62 pagesChapter 2kere evaNo ratings yet
- F4 PHYSICS - LENSES RevisionDocument16 pagesF4 PHYSICS - LENSES RevisionKalarajanNo ratings yet
- Concrete - Lecture 2 - Design of Rectangular Singly-Reinforced BeamDocument28 pagesConcrete - Lecture 2 - Design of Rectangular Singly-Reinforced Beammeh meh100% (1)
- Organic Photochemistry and Pericyclic Reactions Prof. N. D. Pradeep Singh Department of Chemistry Indian Institute of Technology KharagpurDocument19 pagesOrganic Photochemistry and Pericyclic Reactions Prof. N. D. Pradeep Singh Department of Chemistry Indian Institute of Technology KharagpurSandipan SahaNo ratings yet
- DesignBuilder 2.1 Users-Manual LTRDocument642 pagesDesignBuilder 2.1 Users-Manual LTREsteban Omar Bugueño Lara100% (1)
- Astm b117 Testing v1Document3 pagesAstm b117 Testing v1maddumaNo ratings yet
- Chapter 1 Electrons and Holes in Semiconductors: 1.1 Silicon Crystal StructureDocument16 pagesChapter 1 Electrons and Holes in Semiconductors: 1.1 Silicon Crystal StructurepurseyNo ratings yet
- Sheet 3 Solution 2012Document35 pagesSheet 3 Solution 2012jp2away68No ratings yet
- Fluid Flow Through PipesDocument6 pagesFluid Flow Through PipesShardul KaleNo ratings yet
- Lathe Oil and Grease PlanDocument7 pagesLathe Oil and Grease PlanScribdTranslationsNo ratings yet
- Optical and Photochemical Properties of Nonstoichiometric Cadmium Sulfide Nanoparticles: Surface Modification With Copper (II) IonsDocument8 pagesOptical and Photochemical Properties of Nonstoichiometric Cadmium Sulfide Nanoparticles: Surface Modification With Copper (II) IonszahidNo ratings yet
- Vumii App Note Camera Performance CriteriaDocument2 pagesVumii App Note Camera Performance CriteriavlavanyakumarNo ratings yet