Chromatography 2020
- 1. Marwa Fayed, PhD Lecturer of Pharmacognosy Faculty of Pharmacy University of Sadat City
- 2. HISTORY Chromatography (from Greek: chromatos -- color , "graphein" -- writing) • 1903 Tswett - plant pigments separated on chalk columns • 1931 Lederer & Kuhn - LC of carotenoids • 1938 TLC and ion exchange • 1950 Reverse phase LC • 1954 Martin & Synge (Nobel Prize) • 1959 Gel permeation • 1965 instrumental LC (Waters)
- 3. Importance Chromatography has application in every branch of the physical and biological sciences 12 Nobel prizes were awarded between 1937 and 1972 alone for work in which chromatography played a vital role
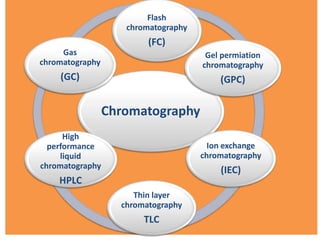
- 4. Chromatography Flash chromatography (FC) Gel permiation chromatography (GPC) Ion exchange chromatography (IEC) Thin layer chromatography TLC High performance liquid chromatography HPLC Gas chromatography (GC)
- 5. Chromatography is a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary while the other moves in a definite direction Definition Stationary phase Mobile phase Solid Liquid (supported on a solid or gel) Liquid Gas
- 6. Theoretical bases of chromatography Distribution equilibrium: -Substances are eluted from the chromatographic column in inverse order of their distribution coefficients with respect to the stationary phase. -The distribution of solutes between two phases is governed by an equilibrium constant known as distribution coefficient, K (or partition coefficient in certain types of chromatography).
- 7. Larger K value K = Cstationary / Cmobile Cstationary = concentration of solute in stationary phase Cmobile = concentration of solute in mobile phase More time is spent in the stationary phase Smaller K value The solute will be eluted very fast with the mobile phase
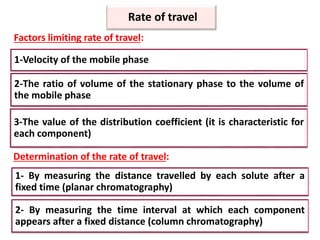
- 8. 1-Velocity of the mobile phase 2-The ratio of volume of the stationary phase to the volume of the mobile phase 3-The value of the distribution coefficient (it is characteristic for each component) Rate of travel Factors limiting rate of travel: Determination of the rate of travel: 1- By measuring the distance travelled by each solute after a fixed time (planar chromatography) 2- By measuring the time interval at which each component appears after a fixed distance (column chromatography)
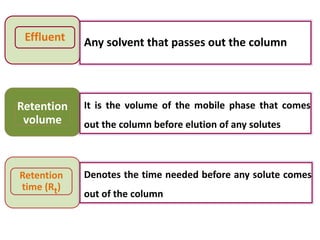
- 10. Development It is the process by which chromatographic separation takes place by allowing the mobile phase to pass through the stationary phase Elution Removal of adsorbed solutes from stationary phase using Eluent Eluate Fraction of the mobile phase that comes out the column containing the solutes
- 11. Effluent Any solvent that passes out the column Retention volume It is the volume of the mobile phase that comes out the column before elution of any solutes Retention time (Rt) Denotes the time needed before any solute comes out of the column
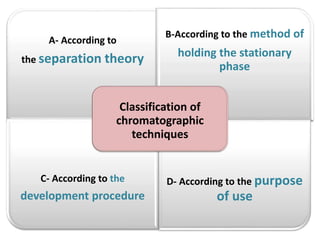
- 13. A- According to the separation theory B-According to the method of holding the stationary phase C- According to the development procedure D- According to the purpose of use Classification of chromatographic techniques
- 14. A- According to the mechanism of separation (separation theory) Adsorption Partition Ion exchange Molecular exclusion ( Gel permeation , Gel filtration) Affinity chromatography
- 15. i- Planar chromatography ii- Columnar chromatography
- 16. i- Ascending development iv- Horizontal development ii- Descending development iii- Radial development
- 17. Analytical chromatography (qualitative and quantitative chromatography) Preparative chromatography (isolation of sample components)
- 18. Adsorption chromatography Adsorption is a surface phenomenon adsorptive (solute) Sorbent (St.phase) In adsorption, the concentration at the interface solid stationary phase/ mobile phase is higher than in the surrounding medium More polar molecules, will be more strongly adsorbed (eluted more slowly)
- 19. More polar molecules, more strongly adsorbed (eluted more slowly) less polar molecules, less adsorbed (eluted more rapidly) Mobile phase Stationary phase sample Cotton or glass wool Eluent Effluent
- 20. stationary phase is Solid Liquid mobile phase Gas mobile phase Liquid Solid chromatography LSC TLC HPLC Gas solid chromatography GSC
- 21. ii-Common adsorbents (Stationary phase) Alumina silica magnesium oxide Charcoal calcium carbonate
- 22. Alumina Commercial alumina is available as: neutral, basic or acid alumina Used for separation of sterols and vitamins Not used for separation of phenolics or carboxylic acids
- 23. Silica gel or Silicic acid The adsorptive properties depend on the hydroxyl groups attached to silicon atoms which interact with polar or unsaturated molecules by hydrogen bonding Not heated silica (not activated) have variable amounts of physically adsorbed water. It acts by partition Heating silica removes this water without loss of surface hydroxyls. This treatment gives adsorbent of maximum activity Used for column and TLC
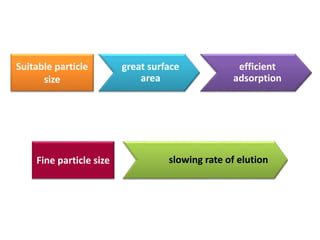
- 24. The Ideal adsorbent must fulfill the following requirements: 1- Selection of the adsorbent (stationary phase) Inert to the solutes Colourless Suitable particle size Insoluble in the mobile phase
- 25. Fine particle size slowing rate of elution Suitable particle size great surface area efficient adsorption
- 26. Strong eluents (more polar solvents) decrease adsorption while weak eluents increase it. Elution of the column can be carried out using solvents of increasing polarity Mobile phase: i-Selection of the solvent:
- 27. A B CMobile phase A: is a solvent (not adsorbed) Mobile phase B: is a developer more affinity to the adsorbent than the solvent but less than the solutes (it allows the solutes to move) Mobile phase C: Eluent It has more eluting power than the developer and can take solutes out of the surface of the adsorbent. D Mobile phase D: Displacer It is the strongest mobile phase and is more strongly adsorbed than solutes.
- 28. Factors affecting column efficiency and chromatographic separation • Decreasing the particle size of the adsorbent results in good separation, but very small particles will offer considerable resistance to flow 1-Particle size of the supporting medium • The column efficiency is improved as the length /width ratio of the column is increased 2-Column dimensions • Irregular packing results in uneven and irregular movement of solvent front and less uniform zone formation 3-Uniformity of packing of the column
- 29. • As temperature is increased, the elution speeds up as the adsorption is generally reduced at higher temperatures 4-Column temperature • Uniform and low flow rate will result in a satisfactory separation and more uniform zone formation than a fast flow rate 5-Solvent flow rate • It is not recommended to interrupt the experiment and continue later 6-Constancy of flow
- 30. • The flow rate is inversely proportional to the viscosity, low viscosity solvents result in high efficiency separation 7-Selection of solvents • Highly concentrated solution results in rapid movement through the column 8-Concentration
- 31. Packing of the column
- 32. 1. Wet packing The adsorbent + the first solvent to be used in the separation =(slurry), is gradually added to the column. The packing is allowed to settle between additions The suspending liquid is allowed to flow out slowly (the liquid level in the column is maintained above the packing at all times) Advantages: Homogenous columns Disadvantages: Air bubbles formation and uneven separation of zones
- 33. 2. Dry packing The adsorbent is added in portions with vibrations between additions. The process is repeated until the column is adequately filled The column is then washed carefully with the first solvent
- 34. iv.Detection of the sample components On-column detection Examination of the separated zones on the column in either • visible light (for colored substances) • or in UV light (for fluorescent compounds) Out-of column detection Collecting the eluate in fractions from the whole column, then testing each of the eluted fractions for the presence of the components. This can be carried out by using • Thin layer (TLC) • or paper chromatography (PC). • The process is called monitoring the fractions
- 35. Applications Isolation and purification of: • vitamins and hormones • alkaloids of Cinchona, Ergot, Opium and Nux- vomica • cardiac glycosides from Digitalis • anthraquinones from Senna species Purification of tincture of alkaloids from pigments before determination of their alkaloidal content
- 37. Partition chromatography The separation of the components of a mixture is dependent on differences in the partition coefficients of the components between two immiscible liquids (or liquid and gas) The liquid stationary phase is adsorbed on an inert support, which may be either packed in a chromatographic tube (column partition chromatography) or layered on a glass plate (TLC) or in the form of sheets of paper (PC) Inert support Liquid st. phase
- 38. stationary phase may be Liquid mobile phase Liquid / Liquid Chromatography (LLC) which flows through the stationary phase in a counter-current manner liquid Gas Gas mobile phase Gas / Liquid Chromatography (GLC) which flows through the stationary phase in a counter-current manner
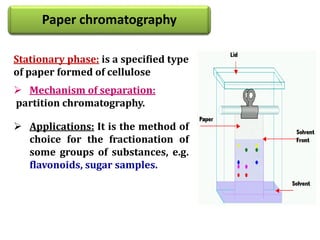
- 39. Paper chromatography Stationary phase: is a specified type of paper formed of cellulose Mechanism of separation: partition chromatography. Applications: It is the method of choice for the fractionation of some groups of substances, e.g. flavonoids, sugar samples.
- 40. Paper chromatography of sugar samples
- 41. G X L U After drying and spraying Retardation factor: It is the ratio between the distance traveled by solute to that traveled by the solvent (Rf). It has values less than one.
- 42. General techniques for paper chromatography i. Ascending technique ii. Descending techniques
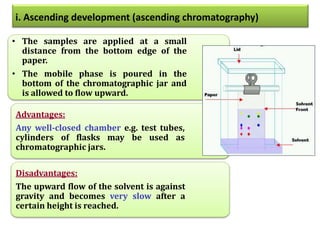
- 43. • The samples are applied at a small distance from the bottom edge of the paper. • The mobile phase is poured in the bottom of the chromatographic jar and is allowed to flow upward. Advantages: Any well-closed chamber e.g. test tubes, cylinders of flasks may be used as chromatographic jars. Disadvantages: The upward flow of the solvent is against gravity and becomes very slow after a certain height is reached. i. Ascending development (ascending chromatography)
- 44. ii. Descending technique The paper is held with its upper end in the solvent trough and passes upward and over an, which suspends it away from the edges of the trough. The other longer end hangs free in the jar. The starting line is situated at 1 to 1.5 cm below the glass rod The solvent is allowed to flow down the paper till its front becomes near the lower end Advantages: There is no gravity resistance to flow, as it is downward
- 45. Thin layer chromatography It is a type of planar chromatography (such as paper chromatography) A thin layer of finely divided adsorbent supported on a glass, plastic or aluminium sheet is used Separation of plant pigments
- 46. Applications Mechanism of separation Separation of lipophilic compounds e.g. terpenoids,steroids and alkaloids It is based on the two mechanisms of chromatographic separation: adsorption and partition
- 47. 1-Inexpensive ( c.f. instrumental analysis). 4-Flexibility of choice of mobile, stationary phases, spray reagents (conc. and drastic reagents can be used)2-Requires little training or knowledge of chromatography 3-Easy scale-up to preparative mode with quick isolation of milligrams to gram amount 5-A large number of samples may be analyzed or separated simultaneously Advantages of TLC
- 48. Spot detection (location or visualization) 1-Physical methods visualization of the spots in the visible or UV light 2-Chemical methods spraying with certain reagents coloured or fluorescent spots
- 49. Iodine solution or vapor or modified Dragendroff's reagent for Alkaloids Aluminum chloride for Flavonoids Antimony trichloride in CHCl3 for Steroids Alkali for anthraquionone derivatives Ninhydrin for Amino acids Ferric chloride for Phenols Spray reagents
- 50. Gel Chromatography This type of chromatography lacks an attractive interaction between the stationary phase and solute. It separates the molecules according to their size. The molecules are eluted in the order of decreasing particle size smaller molecules enter into the gel pores, pass slowly. larger molecules pass through the column at a faster rate than the smaller ones. Mechanism of separation
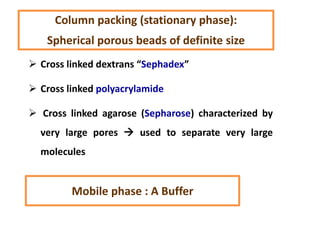
- 51. Cross linked dextrans “Sephadex” Cross linked polyacrylamide Cross linked agarose (Sepharose) characterized by very large pores used to separate very large molecules Mobile phase : A Buffer Column packing (stationary phase): Spherical porous beads of definite size
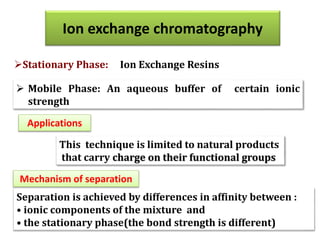
- 52. This technique is limited to natural products that carry charge on their functional groups Separation is achieved by differences in affinity between : • ionic components of the mixture and • the stationary phase(the bond strength is different) Ion exchange chromatography Mechanism of separation Applications Stationary Phase: Ion Exchange Resins Mobile Phase: An aqueous buffer of certain ionic strength
- 54. According to the nature of the solid support According to the nature of the charged groups Polystyrene Cation exchangeAnion exchange weak strong weak strong 3ry amines Quaternary ammonium salts Carboxylic acids Sulfonic acid resin Properties of ion exchangers ( Resins ) Cellulose Dextrans
- 56. Gas chromatography is a chromatographic technique that can be used to separate volatile organic compounds Gas chromatography GC instruments
- 57. 1. The injection port 2. Columns Packed columns Capillary columns
- 58. 4. Stationary phases PEG , polydiethyl siloxane 3. Mobile phases He , N2 , Argon 5. Detectors FID , FPD , ECD , TCD
- 59. Electron capture detector (ECD) Flame ionization detector (FID) Detectors Application Halides, nitrates, nitriles, peroxides, anhydrides, organometallics Most organic compounds Application
- 60. Photoionization detector (PID) Thermal conductivity detector (TCD) Flame photometric detector (FPD) Application Application Application Universal (non destructive) Sulphur, phosphorus, tin, boron, arsenic Aliphatics, aromatics, ketones, esters, aldehydes, amines
- 61. Example of GC chromatogram Chromtogram: It Is a plot of detector signal (absorbance, fluorescence, refractive index etc… versus retention time of solutes in a chromatographic column. The area under the peak is related to solute concentration i.e. essential for quantitative analysis).
- 62. High performance liquid chromatography (HPLC( HPLC is widely used separation technique for analysis and isolation of bioactive natural products. Applications
- 63. HPLC columns Various columns that are secondary to the separating column
- 65. Stationary phases • Normal-phase partition chromatography • Reverse-phase partition chromatography • Ordinary Stationary phase as silica or alumina
- 66. Different modes of mobile phase run • Constant mobile phase composition • The strength (polarity) of the mobile phase is increased in increments by raising the amount of organic solvent fraction.
- 67. HPLC detectors UV/visible Refractive index Fluorescent ConductivityElectrochemical
- 68. Advantages of HPLC The versatility of stationary phases made it popular method for bioassay-guide isolation Run samples, print out chromatograms and spectra automatically Different mode of mobile phase run (gradient, isocratic) Can range-up to preparative scale
- 69. It can be used for the majority of natural compounds that are soluble in organic solvents and can be adapted to ion exchange for isolation of highly polar compounds Excellent separating power Reproducibility of results Advantages of HPLC (cont.)
- 70. It is expensive ( preparative one is more expensive) Preparative column have short life Needs high purity solvents (HPLC grade) Disadvantages of HPLC
- 71. Example of HPLC application for separation of natural products:
Editor's Notes
- Viscosity