Fuel Cells
- 1. Fuel Cells Ankit Saxena
- 2. The Fuel Cell A Need for Change Battery technologies are not keeping pace with demand Battery life Miniaturization Global warming A Possible Solution Fuel Cell: A device that uses hydrogen (or a hydrogen-rich fuel) and oxygen to create an electric current
- 3. What Fuel Cells Do Combustion engines like the turbine and the gasoline engine burn fuels and use the pressure created by the expansion of the gases to do mechanical work. Batteries converted chemical energy back into electrical energy when needed. Fuel cells should do both tasks more efficiently
- 4. How Fuel Cells Work Hydrogen molecules are delivered to the anode side of the fuel cell Electrons are stripped from the molecules and forced to travel through a circuit, producing electricity The leftover protons pass through the fuel cell and are recombined with the lost electrons on the cathode side, producing water molecules
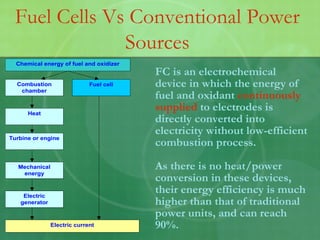
- 5. FC is an electrochemical device in which the energy of fuel and oxidant continuously supplied to electrodes is directly converted into electricity without low-efficient combustion process. As there is no heat/power conversion in these devices, their energy efficiency is much higher than that of traditional power units, and can reach 90%. Fuel Cells Vs Conventional Power Sources
- 6. How much hydrogen? Transportation: gasoline yields 40 kJ/g average annual use: 500 gallons (4000 kg) energy use in one year: 1.6 10 8 kJ H 2 yields 250 kJ/g, need 640 kg H 2 Electricity: average annual energy use: 1.5 10 7 kJ H 2 yields 250 kJ/g, need 60 kg H 2 EACH PERSON IN US NEEDS ~700 KG OF H 2 !!
- 7. Sources of Hydrogen Hydrocarbons: Oxidation: CH 4 + 2 O 2 CO 2 + 2 H 2 O Still produces CO 2 !! Syn-gas: 2 CH 4 + O 2 2 CO + 4 H 2 Produces CO that must be converted to CO 2 !! Electrolysis of water remember H 2 + O 2 H 2 O, E = 1.23 V can be reversed H 2 O H 2 + O 2 Requires large amounts of energy!!
- 8. What does that mean? Water waste Electricity Supply of Hydrogen
- 9. A Timeline: Fuel Cell Implementation 1993: Ballard Power Systems launches first proof-of-concept hydrogen fuel cell bus, Vancouver 1996: Daimler Benz and Toyota are first major companies to unveil prototype fuel cell-powered passenger cars 2000: Ballard Power Systems unveils world’s first production- ready fuel cell for automotive use 2001: Honda opens the first hydrogen production and fueling station in Torrance, CA 2004: The World’s first fuel cell-powered submarine undergoes deep-water trials MTI Micro Fuel Cells introduces a proof-of-concept fuel cell that runs handheld devices 1993: Ballard Power Systems launches first proof-of-concept hydrogen fuel cell bus in Vancouver 1970: Karl Kordesch builds the first practical fuel cell car 1965: NASA uses alkaline fuel cell in Apollo space missions Late 1950’s: Allis-Chambers Manufacturing Co. demonstrates 20 hp fuel cell-powered tractor 1839: Sir William Grove invents the first fuel cell
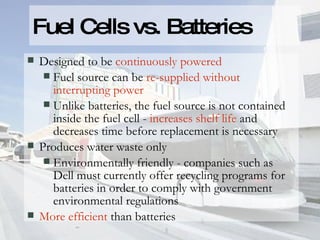
- 10. Fuel Cells vs. Batteries Designed to be continuously powered Fuel source can be re-supplied without interrupting power Unlike batteries, the fuel source is not contained inside the fuel cell - increases shelf life and decreases time before replacement is necessary Produces water waste only Environmentally friendly - companies such as Dell must currently offer recycling programs for batteries in order to comply with government environmental regulations More efficient than batteries
- 11. Benefits of Fuel Cells Environmental Reasons Driving force Lowered emissions Less noisy Higher quality Can be programmed for 99.999% uptime More reliable On site No movable parts
- 12. More Benefits Increased Efficiency Up to 80% with pure Hydrogen With reformer about ~24-32% Gasoline ~20% Battery-Powered ~26% with recharging Distributed Generation Flexible Technology Portable Modular
- 13. Some Limitations Hydrogen: Not readily available, must use other energy sources to convert Infrastructure not in place Difficult to store/distribute High Capital Cost Non-technical barriers Could have dramatic impact
- 14. Oil Industry & Hydrogen Shell Royal Dutch Group $13 billion in capital investments for oil production and exploration, while only having $243 million invested in all alternative energies combined. Often recognized as a leading alternative energy conscious oil company. Why is Shell involved in alternative energies?
- 15. Automotive Industry & Hydrogen GM and others are looking to capitalize on the next generation of cars After enormous success of rivals like Honda and Toyota with hybrid vehicles GM, along with other domestic automotive makers Face serious threats of bankruptcy, making a large R&D push difficult. Rivals such as Toyota and Honda are already ahead of GM and Ford
- 16. A 30 ft. Hydrogen Fuel cell powered transit bus made by Ballard Power Systems in Canada. It has a 275 horsepower engine, and a range of 250 miles before requiring refueling. The only emission from this bus is warm, moist air .
- 17. Honda FCX Operates between -20 C and 95 C. Honda has also built a hydrogen production and filling station prototype .
- 18. Ford FCV Driving range 100 miles. H 2 Fuel Cell
- 19. Ford H2RV H 2 Internal Combustion Engine hybrid with electric motor. Driving Range 125 miles.
- 20. Future 2015 – 2025- Substantial markets for hydrogen-powered vehicles likely to start developing 2020: 5 to 10 million hydrogen-powered cars 2030: 50 million hydrogen powered cars 2040: 150 million hydrogen-powered cars