Bio hydrozen
- 2. Contents Introduction Hydrogen properties Hydrogen production methods Bio hydrogen & its History production methods Economics Conclusion Reference 2
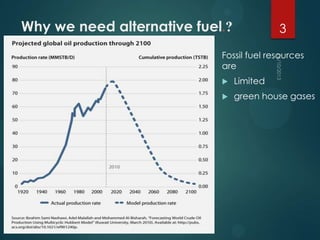
- 3. Why we need alternative fuel.? 3 Fossil fuel resources are Limited green house gases
- 4. 4
- 5. Understanding the hydrogen5 Hydrogen is the first element on the periodic table, making it the lightest element on earth. 0.00005% in air It rises in the atmosphere and is therefore rarely found. pure hydrogen gas, burning in air, producing water and heat. Combustion heat enables hydrogen to act as a fuel.
- 6. Hydrogen properties Colorless and odorless Extremely reactive with oxygen and other oxidizers. Low ignition energy. High flame temperature. Invisible flame in daylight conditions. Small molecular size promotes leaks and diffusion. The cryogenic liquid at 20K is even colder than frozen nitrogen, oxygen or argon. 6
- 7. Key facts about Hydrogen as a fuel 7 Highly combustible and can be used as a fuel. 1g of combustion provides 30000 cals as compared to gasoline that gives only 11000 cals. Can be produced from water using Biological agents. Biologically produced hydrogen is known as Biohydrogen.
- 8. Hydrogen Production 1. Electrolysis. 2. Steam-methane reforming process. 3. Biological process(bio-hydrogen). Hydrogen production always requires more energy than can be retrieved from the gas as a fuel later on when they are produced by above two process. 8
- 9. Simple setup for demonstration of electrolysis of water. 9
- 10. Biological production 10 Biological hydrogen production stands out as an environmentally harmless process carried out under mild operating conditions, using renewable resources. Several types of microorganisms such as the photosynthetic bacteria, cyano bacteria, algae or fermentative bacteria are commonly utilized for biological hydrogen production.
- 11. Milestones 11 discovered that algae can switch between producing O2 and H2. 1939 Hans Gaffron 1997 prof. Anastasios Malis discovered 2006 that deprivation of sulphur will cause the algae to switch from producing H2.He found that enzyme hydrozenase responsible for the reaction. Researcher from the University of Bielfeld have genetically changed the single cell Chlamydomonas reinhardtiiin in such a way that it produces an large amount of hydrogen.
- 12. 12 2007 It was discovered that if cupper is added to block O2 generation in algae. 2007 prof. Anastasios Malis studying solar to chemical energy conversion efficiency in tax X mutants of Chlamydomonas reinhardtiiin , achieved 15% efficiency .
- 13. Methods of Bio hydrogen Production 1.Dark Fermentation 2.Photo Fermentation 3.Combined Fermentation 4.Direct Photolysis (algae) 5.Indirect Photolysis (cynobacteria) 13
- 14. 1. Dark Fermentation 14 Fermentative conversion of organic substrate to biohydrogen. This method doesn’t require light energy. The Gram+ve bacteria of Clostridium genus is of great potential in biohydrogen production. Require wet carbohydrate rich biomass as a substrate. Produces fermentation end product as organic acids, Co2 along with biohydrogen. C6H12O6 + 2H2O 2CO2 2CH3COOH + 4H2 +
- 15. 15 Glu pyruvate acetylcoA fd H2 Carbohydrate mainly glucose is preffered. Pyruvate the product of glucose catabolism is oxidized to acetyl-coA requires ferrodoxin reduction. Reduced ferrodoxin is oxidized by hydrogenase which generates ferrodoxin and release electron as a molecular hydrogen.
- 16. Advantages 16 It produces valuable metabolites as a butyric acid, propionic acid. It is an anaerobic process so no oxygen limitation. It can produce carbon during day and night. Variety of carbon sources can be used as a substrate.
- 17. Drawbacks 17 Relatively lower achievable yield of H2, as a portion of substrate is used to produce organic acids. Anaerobes are incapable of further breakdown of acids. Accumulation of this acids cause a sharp drop of culture pH and subsequent inhibition of bacterial hydrogen production. Product gas mixture contains Co2 which has to be separated.
- 18. Approaches to overcome 18 Metabolic shift of biochemical pathway to arrest the formation of acid and alcohol. To improve the techniques for the seperation of the gases.
- 19. 2.Photo Fermentation 19 Purple non sulphur bacteria genus rhodobacter holds significant promise for production of hydrogen. Photo fermentation where light is required as a source of energy for the production of hydrogen by photosynthetic bacteria. Organic acids are preferred as a substrate. The light energy required in this process is upto the range of 400nm.
- 20. Mechanism 20 CH3COOH + 2H2 + Light 4H2 + 2Co2 Production of hydrogen by photosynthetic bacteria takes place under illumination and in the presence of inert and anaerobic atmosphere for the breakdown of organic substrate to produce hydrogen molecules.
- 21. Advantages Relatively higher achievable yield of H2, as a portion of substrate is used to produce organic acids. Anaerobes are capable of further breakdown of acids in to biohydrogen. Drawbacks It can produce carbon during day only. 21
- 22. Combined fermentation 22 The combination of dark and photo fermentation provides an integrating system for maximization of an hydrogen yield. The idea of combined fermentation takes into an consideration the very fact of relatively lower achievable yield of H2 in dark fermentation. The non utilization fermentation. of acid produced in dark
- 23. Mechanism 23 Stage 1 :- Dark fermentation: Anaerobic fermentation of carbohydrate produces intermediates such as low molecular weight organic acids and Co2 along with hydrogen.
- 24. Stage 2:- Light fermentation The low mol wt organic acid in stage 1 are converted to hydrogen by photosynthetic bacteria. 2CH3COOH + 4H2o CH3COOH + 2Co2 + 4H2 24
- 25. Advantages 25 Two stage fermentation can improve the overall yield of hydrogen and overcomes the major limitation of dark fermentation. Drawbacks:Relatively new approach techno economic feasibility is yet to studied
- 26. 4.Direct Photolysis 26 Certain green algae produces H2 under anaerobic condition. Under deprived of S green algae Chlamydomonas reinhardtiiin become anaerobic in light & commence to synthesis of hydrogen.
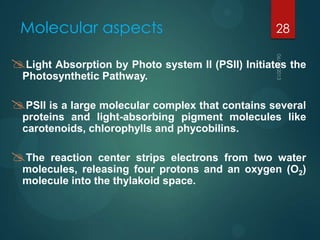
- 28. Molecular aspects 28 Light Absorption by Photo system II (PSII) Initiates the Photosynthetic Pathway. PSII is a large molecular complex that contains several proteins and light-absorbing pigment molecules like carotenoids, chlorophylls and phycobilins. The reaction center strips electrons from two water molecules, releasing four protons and an oxygen (O2) molecule into the thylakoid space.
- 29. 29 The electron carrier from PSII passes through the thylakoid membrane and transfers its electrons to the cytochrome complex, which consists of several subunits including cytochrome f and cytochrome b6. A series of redox reactions within the complex ultimately transfer the electrons to a second electron carrier i.e. photo system I (PSI). As electrons are transported through the complex, protons (H+) outside the thylakoid are carried to the inner thylakoid space.
- 30. 30 Light Absorption by PSI Excites Electrons and Facilitates Electron Transfer to an Electron Acceptor Outside the Thylakoid Membrane. Light absorbed by the PSI reaction center energizes an electron that is transferred to ferredoxin (Fd), a molecule that carries electrons to other reaction pathways outside the thylakoid. The reaction center replaces the electron transferred to ferredoxin by accepting an electron from the electron-carrier molecule that moves between the cytochrome and the PS1
- 31. 31 Under Certain Conditions, Ferredoxin can Carry Electrons to Hydrogenase. Normally, ferredoxin shuttles electrons to an enzyme that reduces NADP+ to NADPH, an important source of electrons needed to convert CO2 to carbohydrates in the carbon-fixing reactions. Under anaerobic conditions, hydrogenase can accept electrons from reduced ferredoxin molecules and use them to reduce protons to molecular hydrogen (H2). 4H+ + ferredoxin(oxi) ――› ferredoxin(reduced)
- 32. H co2 o2 Sunlight Algae production Bioreactor (Light Aerobic) Algae Concentra tor and adapter (DarkAnaerobic ) A L G A E 32 Sunlight H2 Photobioreactor (light anerobic) H2 H2 Nutrient recycle Algae Recycle Fig:- Schematic of Hydrogenase mediated Biophotolysis process
- 33. Economics The US department of energy has targeted a selling price of $2.60/kg as goal for making renewable hydrogen economically viable. 1kg is approximately the energy equivalent to a gallon of gasoline. To achieve this , the efficiency of light to hydrogen conversion must reach 10% while current efficiency is only 1% and selling price is estimated at 13.53/kg. 33
- 34. Reference Hand book of bioenergy and biofuels – V K mutha Journal on Bio hydrogen production aspotential energy resources by Kaushik & D Das. Bio biohydrogen – Microbiological production of hydrogen fuel by P C Hallebeck & J R Bennemen 34
- 35. Conclusion 35 Bio hydrogen is fuel of future Areas of research to increase efficiency include developing of oxygen tolerant hydrogenase and increased hydrogen production rates. Research on cost effective production of bio hydrogen for commercialization is required.
- 36. Thank you t 36