CST Review_Atoms and Atomic Structure
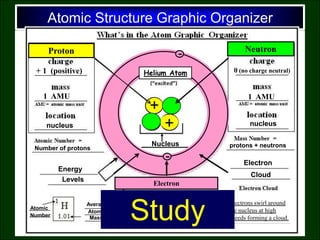
- 3. Atomic Structure Graphic Organizer + + Proton Electron Nucleus - - Energy Levels Neutron - - Helium Atom Electron Cloud + 1 (positive) 0 (no charge neutral) - 1 (negative) 1 AMU 1 AMU 0 AMU Electrons swirl around the nucleus at high speeds forming a cloud. Far from the nucleus Number of protons nucleus nucleus Number Atomic Average Mass Atomic protons + neutrons Study
- 7. 10. What is the reference point? CST Review Questions 21
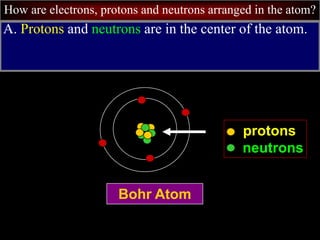
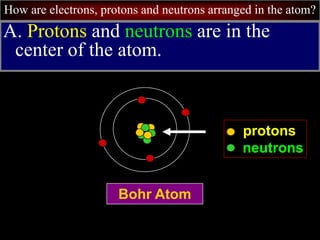
- 8. How are electrons, protons and neutrons arranged in the atom? A. Protons and neutrons are in the center of the atom. Bohr Atom protons neutrons
- 9. How are electrons, protons and neutrons arranged in the atom? Bohr Atom protons neutrons nucleus 1. The center of the atom is called the nucleus.
- 10. How are electrons, protons and neutrons arranged in the atom? B. Electrons swirl around the nucleus at high speeds, forming a cloud. Bohr Atom electrons Electron Cloud
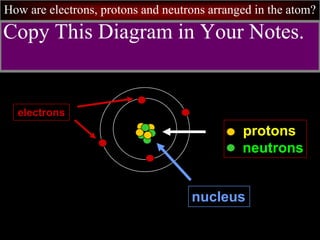
- 11. How are electrons, protons and neutrons arranged in the atom? protons neutrons nucleus Copy This Diagram in Your Notes. electrons
- 12. 10. What is the reference point? CST Review Questions 21
- 13. 10. What is the reference point? CST Review Questions 21
- 20. How are isotopes written? Carbon-13 X. Isotope names contain the name of the element and the mass number of the isotope. Isotope name element mass number
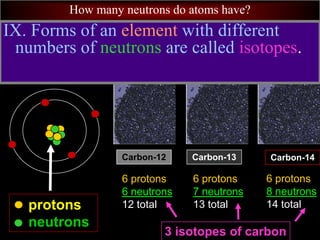
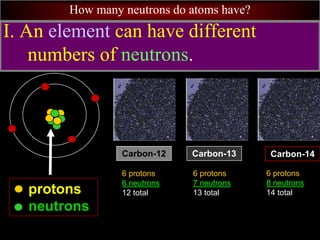
- 21. How many neutrons do atoms have? protons neutrons Carbon-14 6 protons 8 neutrons 14 total Carbon-13 6 protons 7 neutrons 13 total Carbon-12 6 protons 6 neutrons 12 total IX. Forms of an element with different numbers of neutrons are called isotopes. 3 isotopes of carbon
- 22. How many neutrons do atoms have? Carbon-14 6 protons 8 neutrons 14 total Carbon-13 6 protons 7 neutrons 13 total Carbon-12 6 protons 6 neutrons 12 total A. Isotopes are named by the element’s name and mass number (the number of protons + neutrons.) isotopes of carbon
- 25. Think-Group-Share 1. THINK: Read the question and THINK about the answer. 2. GROUP: Discuss the answer with your teammates and agree on the correct answer. 3. SHARE your answers by writing them on the magnetic slate and holding them up.
- 26. Think-Group-Share What is the smallest particle of an element? a molecule an atom a compound
- 27. Think-Group-Share What is the smallest particle of an element? a molecule an atom a compound
- 28. What are the smallest particles of an element? I. Atoms are the smallest particles of an element (building blocks of matter). Nickel Atoms Nickel Coin Nickel Metal Foil
- 29. Think-Group-Share Name the three subatomic particles in the atom. electrons quarks neutrons protons isotopes
- 30. Think-Group-Share Name the three subatomic particles in the atom. electrons quarks neutrons protons isotopes
- 31. What subatomic particles are in the atom? II. Scientists discovered that atoms contain three subatomic particles : - protons - neutrons - electrons. An Oxygen Atom proton neutron electron The Element Oxygen
- 32. Think-Group-Share Where are protons located in the atom? electron cloud energy levels nucleus
- 33. Think-Group-Share Where are protons located in the atom? electron cloud energy levels nucleus
- 34. Think-Group-Share Where are neutrons located in the atom? electron cloud energy levels nucleus
- 35. Think-Group-Share Where are neutrons located in the atom? electron cloud energy levels nucleus
- 36. How are electrons, protons and neutrons arranged in the atom? A. Protons and neutrons are in the center of the atom. Bohr Atom protons neutrons
- 37. How are electrons, protons and neutrons arranged in the atom? Bohr Atom protons neutrons nucleus 1. The center of the atom is called the nucleus.
- 38. Think-Group-Share Where are electrons located in the atom? nucleus energy levels
- 39. Think-Group-Share Where are electrons located in the atom? nucleus energy levels
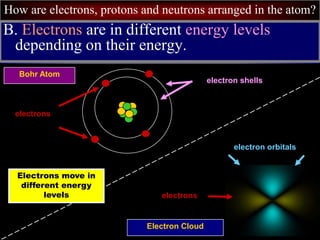
- 40. How are electrons, protons and neutrons arranged in the atom? B. Electrons are in different energy levels depending on their energy. Bohr Atom Electron Cloud electrons electron orbitals electron shells electrons Electrons move in different energy levels
- 41. Think-Group-Share How do electrons move in the atom? A. They do not move. B. They move slowly in the nucleus of the atom. C. They swirl around the nucleus at high speeds.
- 42. Think-Group-Share How do electrons move in the atom? A. They do not move. B. They move slowly in the nucleus of the atom. C. They swirl around the nucleus at high speeds.
- 43. How are electrons, protons and neutrons arranged in the atom? Electrons swirl around the nucleus at high speeds Bohr Atom electrons High Speeds
- 44. Think-Group-Share What do electrons form in the atom? energy levels electron cloud nucleus
- 45. Think-Group-Share What do electrons form in the atom? energy levels electron cloud nucleus
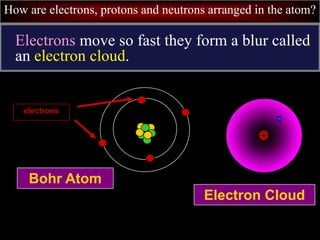
- 46. How are electrons, protons and neutrons arranged in the atom? Electrons move so fast they form a blur called an electron cloud. Bohr Atom electrons Electron Cloud
- 47. Think-Group-Share What is most of the atom? A. the nucleus B. solid C. empty space
- 48. Think-Group-Share What is most of the atom? A. the nucleus B. solid C. empty space
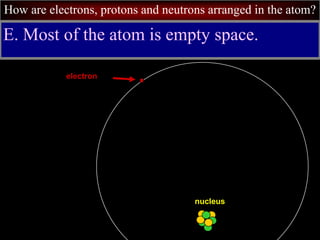
- 49. How are electrons, protons and neutrons arranged in the atom? E. Most of the atom is empty space. nucleus electron
- 50. Think-Group-Share Where is the majority of the MASS of an atom? A. In the electron cloud. B. In the nucleus. C. In the energy levels.
- 51. Think-Group-Share Where is the majority of the MASS of an atom? A. In the electron cloud. B. In the nucleus. C. In the energy levels.
- 52. Think-Group-Share What charge does a proton have? A. positive (+1) B. negative (-1) C. neutral - no charge (0)
- 53. Think-Group-Share What charge does a proton have? A. positive (+1) B. negative C. neutral (no charge)
- 54. Think-Group-Share What charge does a neutron have? A. positive (+1) B. negative (-1) C. neutral - no charge (0)
- 55. Think-Group-Share What charge does a neutron have? A. positive B. negative C. neutral - no charge (0)
- 56. Think-Group-Share What charge does an electron have? A. positive (+1) B. negative (-1) C. neutral - no charge (0)
- 57. Think-Group-Share What charge does an electron have? A. positive (+1) B. negative (-1) C. neutral - no charge (0)
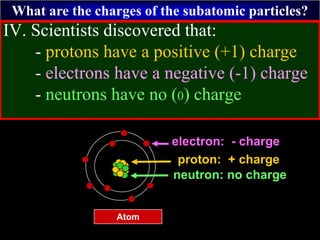
- 58. What are the charges of the subatomic particles? IV. Scientists discovered that: - protons have a positive (+1) charge - electrons have a negative (-1) charge - neutrons have no (0) charge Atom proton: + charge neutron: no charge electron: - charge
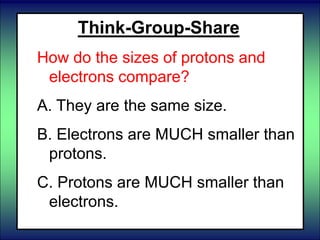
- 59. Think-Group-Share How do the sizes of protons and neutrons compare? A. They are the same size. B. Protons are smaller than neutrons. C. Neutrons are smaller than protons.
- 60. Think-Group-Share How do the sizes of protons and neutrons compare? A. They are the same size. B. Protons are smaller than neutrons. C. Neutrons are smaller than protons.
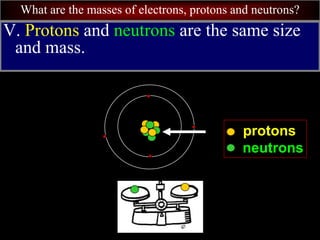
- 61. What are the masses of electrons, protons and neutrons? V. Protons and neutrons are the same size and mass. protons neutrons
- 62. Think-Group-Share How do the sizes of protons and electrons compare? A. They are the same size. B. Electrons are MUCH smaller than protons. C. Protons are MUCH smaller than electrons.
- 63. Think-Group-Share How do the sizes of protons and electrons compare? A. They are the same size. B. Electrons are MUCH smaller than protons. C. Protons are MUCH smaller than electrons.
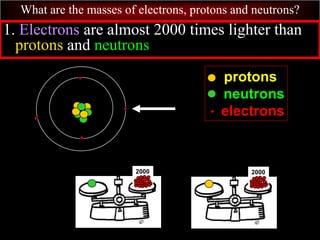
- 64. What are the masses of electrons, protons and neutrons? C. Electrons are MUCH smaller than protons and neutrons. protons neutrons electrons
- 65. What are the masses of electrons, protons and neutrons? 1. Electrons are almost 2000 times lighter than protons and neutrons. protons neutrons electrons 2000 2000
- 66. Think-Group-Share What is the mass of a proton? A. 1 AMU (atomic mass unit) B. 0 AMU (atomic mass unit)
- 67. Think-Group-Share What is the mass of a proton? A. 1 AMU (atomic mass unit) B. 0 AMU (atomic mass unit)
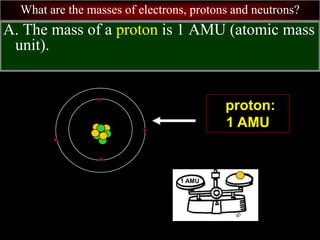
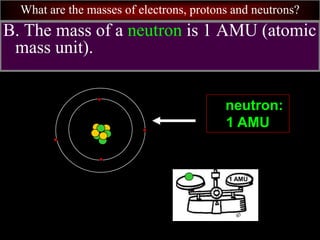
- 68. What are the masses of electrons, protons and neutrons? A. The mass of a proton is 1 AMU (atomic mass unit). proton: 1 AMU 1 AMU
- 69. Think-Group-Share What is the mass of an electron? A. 1 AMU (atomic mass unit) B. 0 AMU (atomic mass unit)
- 70. Think-Group-Share What is the mass of an electron? A. 1 AMU (atomic mass unit) B. 0 AMU (atomic mass unit)
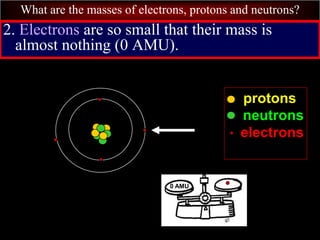
- 71. What are the masses of electrons, protons and neutrons? 2. Electrons are so small that their mass is almost nothing (0 AMU). protons neutrons electrons 0 AMU
- 72. Think-Group-Share What is the mass of a neutron? A. 1 AMU (atomic mass unit) B. 0 AMU (atomic mass unit)
- 73. Think-Group-Share What is the mass of a neutron? A. 1 AMU (atomic mass unit) B. 0 AMU (atomic mass unit)
- 74. What are the masses of electrons, protons and neutrons? B. The mass of a neutron is 1 AMU (atomic mass unit). neutron: 1 AMU 1 AMU
- 75. Think-Group-Share What is atomic number? A. The number of protons + neutrons. B. The number of protons. C. The number of neutrons.
- 76. Think-Group-Share What is atomic number? A. The number of protons + neutrons. B. The number of protons. C. The number of neutrons.
- 77. How many protons do atoms have? B. The number of protons of an element is called the atomic number. Carbon Oxygen 6 protons Atomic Number = 6 8 protons Atomic Number = 8 Hydrogen 1 proton Atomic Number = 1
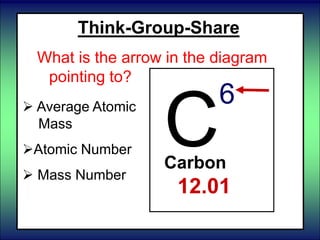
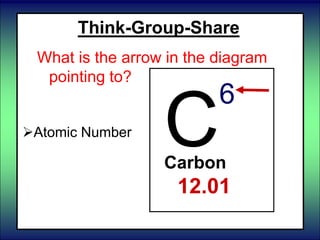
- 78. Think-Group-Share What is the arrow in the diagram pointing to? C 6 Carbon 12.01 Average Atomic Mass Atomic Number Mass Number
- 79. Think-Group-Share What is the arrow in the diagram pointing to? Atomic Mass Atomic Number Mass Number of the most common isotope. C 6 Carbon 12.01
- 80. Think-Group-Share What is the name of the element with an atomic number of 92?
- 81. Uranium Think-Group-Share What is the name of the element with an atomic number of 92?
- 82. Uranium Think-Group-Share What is the atomic number of Barium?
- 83. 56 Think-Group-Share What is the atomic number of Barium?
- 84. 56 Think-Group-Share How many protons does the element Arsenic have?
- 85. 33 Think-Group-Share How many protons does the element Arsenic have?
- 86. Cu Think-Group-Share How many electrons does a neutral atom of Silver have ?
- 87. 47 Think-Group-Share How many electrons does a neutral atom of Silver have ?
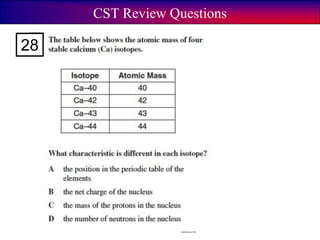
- 89. Think-Group-Share What is mass number? A. The number of protons + neutrons. B. The number of protons. C. The number of neutrons.
- 90. Think-Group-Share What is mass number? A. The number of protons + neutrons. B. The number of protons. C. The number of neutrons.
- 91. How many neutrons do atoms have? A. The sum of neutrons + protons in an element is called the mass number. protons neutrons Carbon-14 6 protons 8 neutrons 14 total (Mass Number) Carbon-13 6 protons 7 neutrons 13 total (Mass Number) Carbon-12 6 protons 6 neutrons 12 total (Mass Number)
- 92. How are isotopes written? Carbon-13 III. Isotope names contain the name of the element and the mass number of the isotope. Isotope name element mass number Draw this
- 93. Think-Group-Share What is the mass number of the isotope Cesium-141?
- 94. Think-Group-Share What is the mass number of the isotope Cesium-141? 141
- 95. Think-Group-Share What is average atomic mass? A. The number of protons + neutrons. B. The number of neutrons. C. The average mass of all of the isotopes.
- 96. Think-Group-Share What is average atomic mass? A. The number of protons + neutrons. B. The number of neutrons. C. The average mass of all of the isotopes.
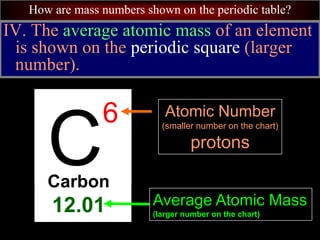
- 97. How are mass numbers shown on the periodic table? A. The average atomic mass is the average mass of all of the isotopes of that element. C 6 Carbon 12.01 Average Atomic Mass (larger number on the chart) Atomic Number (smaller number on the chart) protons
- 98. Think-Group-Share What is the arrow in the diagram pointing to? C 6 Carbon 12.01 Average Atomic Mass Atomic Number Mass Number
- 99. Think-Group-Share What is the arrow in the diagram pointing to? C 6 Carbon 12.01 Average Atomic Mass Atomic Number Mass Number of the most common isotope.
- 100. How are mass numbers shown on the periodic table? IV. The average atomic mass of an element is shown on the periodic square (larger number). C 6 Carbon 12.01 Average Atomic Mass (larger number on the chart) Atomic Number (smaller number on the chart) protons
- 101. Think-Group-Share What is the same about all isotopes of an element? A. They have the same number of neutrons. B. They have the same number of protons. C. They have the same atomic mass.
- 102. Think-Group-Share What is the same about all isotopes of an element? A. They have the same number of neutrons. B. They have the same number of protons. C. They have the same atomic mass.
- 103. How many neutrons do atoms have? I. An element can have different numbers of neutrons. protons neutrons Carbon-14 6 protons 8 neutrons 14 total Carbon-13 6 protons 7 neutrons 13 total Carbon-12 6 protons 6 neutrons 12 total
- 104. Think-Group-Share What is different about the isotopes of an element? A. They have different numbers of neutrons. B. They have different numbers of electrons. C. They have different numbers of protons.
- 105. Think-Group-Share What is different about the isotopes of an element? A. They have different numbers of neutrons. B. They have different numbers of electrons. C. They have different numbers of protons.
- 106. What is an isotope of an element? protons neutrons Carbon-14 6 protons 8 neutrons 14 total Carbon-13 6 protons 7 neutrons 13 total Carbon-12 6 protons 6 neutrons 12 total II. The same element with different numbers of neutrons is called an isotope. 3 isotopes of carbon
- 107. Think-Group-Share What is the isotope name of the atom with 3 protons and 4 neutrons?
- 108. Think-Group-Share What is the isotope name of the atom with 3 protons and 4 neutrons? Lithium-7
- 110. Think-Group-Share What is the isotope name of the atom with 3 protons and 4 neutrons? Lithium-7 Mass Number = 3 protons + 4 neutrons
- 111. How are isotopes written? Carbon-13 Review Isotope name element mass number
- 112. How are isotopes written? C-13 Review Isotope Symbol chemical symbol mass number
- 113. Where are the most common mass numbers shown? Review C 6 Carbon 12.01 Avg Atomic Mass (larger number on the chart) Atomic Number (smaller number on the chart) protons
- 114. Where are the most common mass numbers shown? Review C 6 Carbon 12.01 Atomic Mass (larger number on the chart) Atomic Number (smaller number on the chart) protons