04 carbon and the molecular diversity of life
- 1. LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity of Life Lectures by Erin Barley Kathleen Fitzpatrick © 2011 Pearson Education, Inc.
- 2. Overview: Carbon: The Backbone of Life • Living organisms consist mostly of carbon-based compounds • Carbon is unparalleled in its ability to form large, complex, and diverse molecules • Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds © 2011 Pearson Education, Inc.
- 3. Figure 4.1
- 4. Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms © 2011 Pearson Education, Inc.
- 5. • Vitalism, the idea that organic compounds arise only in organisms, was disproved when chemists synthesized these compounds • Mechanism is the view that all natural phenomena are governed by physical and chemical laws © 2011 Pearson Education, Inc.
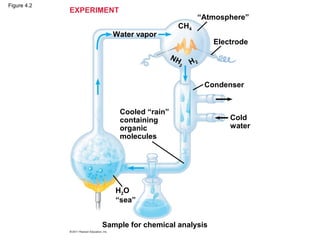
- 6. Organic Molecules and the Origin of Life on Earth • Stanley Miller’s classic experiment demonstrated the abiotic synthesis of organic compounds • Experiments support the idea that abiotic synthesis of organic compounds, perhaps near volcanoes, could have been a stage in the origin of life © 2011 Pearson Education, Inc.
- 7. Figure 4.2 EXPERIMENT “Atmosphere” CH4 Water vapor Electrode NH 3 H2 Condenser Cooled “rain” containing Cold organic water molecules H2O “sea” Sample for chemical analysis
- 8. Carbon atoms can form diverse molecules by bonding to four other atoms • Electron configuration is the key to an atom’s characteristics • Electron configuration determines the kinds and number of bonds an atom will form with other atoms © 2011 Pearson Education, Inc.
- 9. The Formation of Bonds with Carbon • With four valence electrons, carbon can form four covalent bonds with a variety of atoms • This ability makes large, complex molecules possible • In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral shape • However, when two carbon atoms are joined by a double bond, the atoms joined to the carbons are in the same plane as the carbons © 2011 Pearson Education, Inc.
- 10. Figure 4.3 Name and Molecular Structural Ball-and- Space-Filling Comment Formula Formula Stick Model Model (a) Methane CH4 (b) Ethane C2H6 (c) Ethene (ethylene) C2H4
- 11. • The electron configuration of carbon gives it covalent compatibility with many different elements • The valences of carbon and its most frequent partners (hydrogen, oxygen, and nitrogen) are the “building code” that governs the architecture of living molecules © 2011 Pearson Education, Inc.
- 12. Figure 4.4 Hydrogen Oxygen Nitrogen Carbon (valence = 1) (valence = 2) (valence = 3) (valence = 4)
- 13. • Carbon atoms can partner with atoms other than hydrogen; for example: – Carbon dioxide: CO2 O=C=O – Urea: CO(NH2)2 © 2011 Pearson Education, Inc.
- 14. Figure 4.UN01 Urea
- 15. Molecular Diversity Arising from Carbon Skeleton Variation • Carbon chains form the skeletons of most organic molecules • Carbon chains vary in length and shape © 2011 Pearson Education, Inc.
- 16. Figure 4.5 (a) Length (c) Double bond position Ethane Propane 1-Butene 2-Butene (b) Branching (d) Presence of rings Butane 2-Methylpropane Cyclohexane Benzene (isobutane)
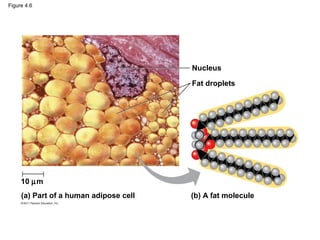
- 17. Hydrocarbons • Hydrocarbons are organic molecules consisting of only carbon and hydrogen • Many organic molecules, such as fats, have hydrocarbon components • Hydrocarbons can undergo reactions that release a large amount of energy © 2011 Pearson Education, Inc.
- 18. Figure 4.6 Nucleus Fat droplets 10 µm (a) Part of a human adipose cell (b) A fat molecule
- 19. Isomers • Isomers are compounds with the same molecular formula but different structures and properties – Structural isomers have different covalent arrangements of their atoms – Cis-trans isomers have the same covalent bonds but differ in spatial arrangements – Enantiomers are isomers that are mirror images of each other © 2011 Pearson Education, Inc.
- 20. Figure 4.7 (a) Structural isomers (b) Cis-trans isomers cis isomer: The two Xs trans isomer: The two Xs are on the same side. are on opposite sides. (c) Enantiomers CO2H CO2H H NH2 NH2 H CH3 CH3 L isomer D isomer
- 21. • Enantiomers are important in the pharmaceutical industry • Two enantiomers of a drug may have different effects • Usually only one isomer is biologically active • Differing effects of enantiomers demonstrate that organisms are sensitive to even subtle variations in molecules © 2011 Pearson Education, Inc.
- 22. Figure 4.8 Effective Ineffective Drug Condition Enantiomer Enantiomer Ibuprofen Pain; inflammation S-Ibuprofen R-Ibuprofen Albuterol Asthma R-Albuterol S-Albuterol
- 23. A few chemical groups are key to the functioning of biological molecules • Distinctive properties of organic molecules depend on the carbon skeleton and on the molecular components attached to it • A number of characteristic groups called functional groups can replace the hydrogens attached to skeletons of organic molecules © 2011 Pearson Education, Inc.
- 24. The Chemical Groups Most Important in the Processes of Life • Functional groups are the components of organic molecules that are most commonly involved in chemical reactions • The number and arrangement of functional groups give each molecule its unique properties © 2011 Pearson Education, Inc.
- 25. Figure 4.UN02 Estradiol Testosterone
- 26. • The seven functional groups that are most important in the chemistry of life: – Hydroxyl group – Carbonyl group – Carboxyl group – Amino group – Sulfhydryl group – Phosphate group – Methyl group © 2011 Pearson Education, Inc.
- 27. Figure 4.9a Hydroxyl STRUCTURE Alcohols NAME OF (Their specific COMPOUND names usually (may be written end in -ol.) HO—) EXAMPLE • Is polar as a result FUNCTIONAL of the electrons PROPERTIES spending more time near the electronegative oxygen atom. Ethanol • Can form hydrogen bonds with water molecules, helping dissolve organic compounds such as sugars.
- 28. Figure 4.9b Carbonyl STRUCTURE Ketones if the carbonyl NAME OF group is within a COMPOUND carbon skeleton Aldehydes if the carbonyl group is at the end of the carbon skeleton EXAMPLE • A ketone and an FUNCTIONAL aldehyde may be PROPERTIES structural isomers with different properties, as is the case for acetone and propanal. • Ketone and aldehyde Acetone groups are also found in sugars, giving rise to two major groups of sugars: ketoses (containing ketone groups) and aldoses (containing aldehyde Propanal groups).
- 29. Figure 4.9c Carboxyl STRUCTURE Carboxylic acids, or organic NAME OF acids COMPOUND EXAMPLE • Acts as an acid; can FUNCTIONAL donate an H+ because the PROPERTIES covalent bond between oxygen and hydrogen is so polar: Acetic acid Nonionized Ionized • Found in cells in the ionized form with a charge of 1– and called a carboxylate ion.
- 30. Figure 4.9d Amino STRUCTURE Amines NAME OF COMPOUND EXAMPLE • Acts as a base; can FUNCTIONAL pick up an H+ from the PROPERTIES surrounding solution (water, in living organisms): Glycine Nonionized Ionized • Found in cells in the ionized form with a charge of 1+.
- 31. Figure 4.9e Sulfhydryl STRUCTURE Thiols NAME OF COMPOUND (may be written HS—) EXAMPLE • Two sulfhydryl groups can FUNCTIONAL react, forming a covalent PROPERTIES bond. This “cross-linking” helps stabilize protein structure. • Cross-linking of cysteines in hair proteins maintains the curliness or straightness Cysteine of hair. Straight hair can be “permanently” curled by shaping it around curlers and then breaking and re-forming the cross-linking bonds.
- 32. Figure 4.9f Phosphate STRUCTURE Organic phosphates NAME OF COMPOUND EXAMPLE • Contributes negative FUNCTIONAL charge to the molecule PROPERTIES of which it is a part (2– when at the end of a molecule, as at left; 1– when located internally in a chain of phosphates). Glycerol phosphate • Molecules containing phosphate groups have the potential to react with water, releasing energy.
- 33. Figure 4.9g Methyl STRUCTURE Methylated compounds NAME OF COMPOUND EXAMPLE • Addition of a methyl group FUNCTIONAL to DNA, or to molecules PROPERTIES bound to DNA, affects the expression of genes. • Arrangement of methyl groups in male and female sex hormones affects their shape and function. 5-Methyl cytidine
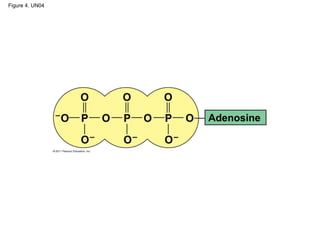
- 34. ATP: An Important Source of Energy for Cellular Processes • One phosphate molecule, adenosine triphosphate (ATP), is the primary energy- transferring molecule in the cell • ATP consists of an organic molecule called adenosine attached to a string of three phosphate groups © 2011 Pearson Education, Inc.
- 35. Figure 4. UN04 Adenosine
- 36. The Chemical Elements of Life: A Review • The versatility of carbon makes possible the great diversity of organic molecules • Variation at the molecular level lies at the foundation of all biological diversity © 2011 Pearson Education, Inc.
Editor's Notes
- Figure 4.1 What properties make carbon the basis of all life?
- Figure 4.2 Inquiry: Can organic molecules form under conditions believed to simulate those on the early Earth?
- Figure 4.3 The shapes of three simple organic molecules.
- Figure 4.4 Valences of the major elements of organic molecules.
- Figure 4.UN01 In-text figure, p. 61
- Figure 4.5 Four ways that carbon skeletons can vary.
- Figure 4.6 The role of hydrocarbons in fats.
- Figure 4.7 Three types of isomers, compounds with the same molecular formula but different structures.
- Figure 4.8 The pharmacological importance of enantiomers.
- Figure 4.UN02 In-text figure, p. 63
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.9 Exploring: Some Biologically Important Chemical Groups
- Figure 4.UN04 In-text figure, p. 66