10.1 The Mole powerpoint.ppt
- 1. 1 Chapter 10 Chemical Quantities 10.2 The Mole Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings
- 2. Section 10.1 The Mole: A Measurement of Matter OBJECTIVES: Describe methods of measuring the amount of something.
- 3. Section 10.1 The Mole: A Measurement of Matter OBJECTIVES: Define Avogadro’s number as it relates to a mole of a substance.
- 4. Section 10.1 The Mole: A Measurement of Matter OBJECTIVES: Distinguish between the atomic mass of an element and its molar mass.
- 5. Section 10.1 The Mole: A Measurement of Matter OBJECTIVES: Describe how the mass of a mole of a compound is calculated.
- 6. 6 Collection Terms A collection term states a specific number of items. • 1 dozen donuts = 12 donuts • 1 ream of paper = 500 sheets • 1 case = 24 cans Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings
- 7. 7 A mole (mol) is a collection that contains • The same number of particles as there are carbon atoms in 12.01 g of carbon. • 6.022 x 1023 atoms of an element (Avogadro’s number). 1 mol C = 6.022 x 1023 C atoms 1 mol CO2 = 6.022 x 1023 CO2 molecules 1 mol NaCl = 6.022 x 1023 NaCl formula units A Mole of Particles
- 8. 8 Samples of One Mole Quantities Table 6.1 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings
- 9. 9 Using Avogadro’s Number Avogadro’s number • Converts moles of a substance to the number of particles. • Converts particles of a substance to moles. Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings
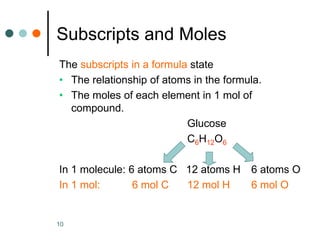
- 10. 10 Subscripts and Moles The subscripts in a formula state • The relationship of atoms in the formula. • The moles of each element in 1 mol of compound. Glucose C6H12O6 In 1 molecule: 6 atoms C 12 atoms H 6 atoms O In 1 mol: 6 mol C 12 mol H 6 mol O
- 11. 11 Subscripts State Atoms and Moles 1 mol C9H8O4 = 9 mol C 8 mol H 4 mol O Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings
- 12. 12 Learning Check A. How many moles O are in 0.150 mol aspirin C9H8O4? B. How many O atoms are in 0.150 mol aspirin C9H8O4?
- 13. 13 Solution A. How many mol O are in 0.150 mol aspirin C9H8O4? 0.150 mol C9H8O4 x 4 mol O = 0.600 mol O 1 mol C9H8O4 subscript factor B. How many O atoms are in 0.150 mol aspirin C9H8O4? 0.150 mol C9H8O4 x 4 mol O x 6.022 x 1023 O atoms 1 mol C9H8O4 1 mol O subscript Avogadro’s factor Number = 3.61 x 1023 O atoms
- 14. 14 Learning Check How many O atoms are in 0.150 mol aspirin C9H8O4?
- 15. 15 Solution How many O atoms are in 0.150 mol aspirin C9H8O4? 0.150 mol C9H8O4 x 4 mol O x 6.022 x 1023 O atoms 1 mol C9H8O4 1 mol O subscript Avogadro’s factor number = 3.61 x 1023 O atoms