5. product certification scheme for chinese materia medica tsang kl
- 1. Product Certification Scheme for Chinese Materia Medica 中藥材的產品認證計劃 Mr. KL Tsang, BBS, JP HKPC 香港生產力促進局 5 September 2013
- 2. Hong Kong Productivity Council 生產力局 • HKPC is a multi-disciplinary organization established by statute in 1967 to promote increased productivity and the use of more efficient methods throughout Hong Kong’s business community. 檢測及認証 生產力局成立於1967年,是政府資助的法定工業支援機構, 致力協助本港製造業、服務業及中小型企業達到卓越的生 產力,以提升競爭力及持續發展能力。 • With about 300 professional consultants and multi-disciplinary 產品開發 expertise, backed by our advanced manufacturing support and over 30 testing facilities & technical centers 生產力局近300位專家顧問,橫跨產品開發、創新及知識產 權、檢測認證、製造科技、生產管理、環境科技、企業管 理及資訊科技等不同專業領域。本局總部大樓更設有30多 環境管理 個實驗室及技術中心。 創新 業務管理 知識產權 品牌
- 3. Hong Kong Productivity Council 生產力局 Over the past 40 years close working with various businesses , HKPC has developed and implemented a large number of certification and recognition schemes : 在過去40年與各行業緊密合作,生產力局完成了大量的認證和認可計劃, 如: 優質旅遊服務計劃 The Quality Tourism Scheme 優質婚禮商戶 Quality Wedding Merchant Scheme 優質魚缸水計劃 The Quality Seawater Accreditation Scheme for the Health, Welfare and Food Bureau 翡翠及鑽石測試認証及標識制度系統The accredited gemological certification and label scheme for diamond and jadeite jade 優質餐飲業環保管理計劃The Quality Restaurant Environmental Management Accreditation Scheme 粵港清潔生產夥伴”標誌計畫GD & HK Joint recognition scheme 香港工商業獎The Hong Kong Awards for Industries 香港環保卓越計劃The Hong Kong Awards for Environmental Excellence for the Environmental Campaign Committee 中小企業優質顧客服務大獎The SME customer Service Excellence Award 創新知識企業獎 The Innovation-Knowledge Enterprise Award 香港無線科技傑出大獎 The Hong Kong Wireless Technology Excellence Awards; etc 香港企業公民嘉許計劃 The Hong Kong Corporate Citizen Award, etc
- 4. Product Certification Scheme for Chinese Materia Medica 中藥材產品認證計劃 • CMM Certification中藥材認證 • CMM Certification Requirement中藥材認證要求 • Benefit of the Certification Scheme認證計劃的裨 益
- 5. What is the Product Certification Scheme for Chinese Materia Medica? 甚麼是中藥材認證? • HKPC is establishing a PCS for CMM initiated by CM Panel of HKCTC and funded by ITF and sponsored by CM trades 香港生產促進局現正制訂一項中藥材產品認證計劃,該項計劃獲香港檢測和 認證局推動中藥行業檢測和認證服務小組支持。有關制訂工作主要由創新及 科技基金的一般支援計劃提供資助,檢測和認證業及中藥業亦有提供贊助 • CMM Certification is a voluntary programme in HK. The certification process includes management audit, documents & records audit, sampling, testing, evaluation , and surveillance in order to certifies the supplier’s capability to provide product in compliance with the standard requirement. 中藥材產品認證計劃是針對本港常用的中藥材的一項自願性的品質認證計劃。 透過一系列認證過程,包括管理程序的審核、文件審核、實地審核、取樣、 化驗測試、結果評價、監督等等,以確認該申證企業具備能力保證供應該中 藥材達到相關標準的要求。
- 6. What is the Standard which the Development of the PCS is Based on? 中藥材產品認證計劃是按甚麼原則或標準來制定? The rules, procedures and requirements set in the PCS are based on related ISO standards, such as ISO Guide 67, ISO 17067, etc . Certification bodies and laboratories involved in the certification activities have to be accredited according to the ISO requirements. 中藥材產品認證計劃的認證規則、程序及管理要求是參考 相關的國際標準而編制, 如ISO Guide 67, ISO 17067 等等。 此外,中藥材認證的執行機構即認證機構及實驗所應取得 認可機構的認可,達到國際標準的要求,才能對中藥材供 應商提供產品認證及化驗服務。
- 7. PCS Development and Subsequent Implementation 中藥材產品認證計劃的建立及日後執行方式 PSC Development 產品認證計劃的建立 HKCTC / Chinese Medicine Panel/ITC/CM Trades • Advice on PCS • Support and Promote the PCS • Funding the project 提供意見, 支持及推廣PCS, 項目支助 CMM Certification 中藥材認證 HKCAS Accredited Certification Body HKPC • ITF project applicant • Develop the PCS • Promote the PCS (項目申請、PCS建構、宣傳) • Provide product certification services for CMM according to the PCS (按PCS提供客戶中藥材產品認證) Clients Project Advisory Committee Review & comment the PCS (在PCS建設過程中提供措指導 及建議) • CMM Manufacturer, Trader, Importer etc • User, Dealer, Buyer etc (需要中藥材產品認證的客戶) HOKLAS Accredited Testing Lab • Provide accredited CMM testing (按港標提供客戶中藥材產品化驗)
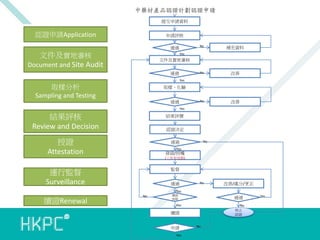
- 8. 中藥材產品認證計劃認證申請 提交申請資料 認證申請Application 申請評核 No 文件及實地審核 Document and Site Audit 補充資料 No 改善 No 通過 改善 Yes 文件及實地審核 通過 Yes 取樣分析 Sampling and Testing 取樣、化驗 通過 Yes 結果評核 Review and Decision 結果評價 授證 Attestation 通過 認證決定 No Yes 發證/授權 (三年有效期) 監督 運行監督 Surveillance 通過 No 改善/處分/更正 Yes 續證Renewal No 續證 到期 通過 Yes No 停止 認證 續證 申請 Yes No Yes
- 9. What Chinese Materia Medica Can be Certified 什麼中藥材可以認證 • Chinese Materia Medica in Hong Kong Standard for Chinese Materia Medica 在香港中藥材標準(港標)內所載的中藥材 • CMM not in HKCMM Std but in the Pharmacopoeia of The People's Republic of China , may also be certified; however, the CMM has to be verified by authentication test, assay, and contaminants analysis; equivalent to those tests in HKCMM Std. 不在港標內,但在中華人民共和國藥典內所載 的中藥材仍然可以按本計劃進行認證,但必須 按規定的測試項目進行化驗,包括藥材鑑定、 品質檢定及污染物測試;如同於《香港中藥材 標準》內所要求。
- 11. Certification Requirement 認證要求 • Traceability of the CMM supply – quality supply source and controlled delivery 中藥材的溯源 – 有品質控制的供應來源及運送過程 • Applicant’s quality management – quality system or procedure for handling the CMM , documents, personnel, and dealing with interested parties, etc 申證機構的品質管理 - 中藥材運作、相關文件、人員、 相關方等等的品質管理系統或程序 • Product requirement – sampling and testing in compliance with the related requirements in HK CMM Standards 產品要求 – 取樣及化驗符合香港中藥材標準內相關要求
- 12. Quality Management 品質管理 Applicants shell develop, operate, and maintain an effective quality management system or procedure that is capable of achieving the consistent fulfillment of the certification requirements, which shell address the following, but are not limited to :申證機構應建立及執行有效的品質管理系統或程序,以確保持續符合認證要求,其內 容應包括:1. CMM procurement & delivery中藥材的採購及運送 2. CMM quality inspection and acceptance conditions中藥材的品質檢查及收貨條件 3. Processing control加工過程控制 4. CMM Storage中藥材的儲存 5. Documentation system文件管理系統 6. Control of documents & record文件及紀錄控制 7. Sale and after sale control銷售及售後控制 8. Recall產品回收 9. Complaint handling投訴處理 10. Subcontracting control 分包控制 11. Internal audit內部審核 12. Training; etc培訓等等
- 13. Product requirement 產品要求 Comply with the related quality and requirements in HK CMM Standard 符合香港中藥材標準的相關 要求,包括:1. CMM Identification中藥材的鑒別 2. Assay of CMM中藥材的指標成份含量 3. Contaminants 污染物分析含量
- 14. CMM Identification 中藥材鑒別 According to HKCMM Standards, the methods for CMM identification including 按《香港中藥材標準》, 中藥材可經由一些檢測方法來進行鑒別,包括: • CMM description : outlook, shape, color, smell, hardness etc.性狀鑒別 : 中藥材的外觀、形狀、 色澤、氣味、硬度等等 • Microscopic identification : such as cross section inspection 顯微鑒別 : 如中藥材橫斷面所呈現的 特性,細胞種類、形狀及排列等等 • Fingerprint chromatogram, the specific chemicals in the CMM showing on the chromatogram 指紋圖譜 : 中藥材內所含有的特殊化學品,經個 別分離及偵測後,成為該藥材特有指紋 Comparing the collected results or/and figures with the Standards, helps to identify the CMM.將所收集的 數據及圖樣,比對標準結果,如《香港中藥材標 準》,即可以科學及客觀的方法鑒別中藥材。 三七的顯微圖及指紋圖譜
- 15. Assay of CMM 甚麼是中藥材的指標成份 In addition to the impurity, contaminant, water content etc, active ingredient or assay marker of a CMM may indicate the quality. For an example Ginsenoside Rb1, Ginsenoside Rg1, and Notoginsenoside R1 in Radix Notoginseng. By using a HPLC in a laboratory, it can determine the assay quantity in compliance with the standard limit. 除了中藥材的雜質、污染物量、水分等含量影響品質外,每種中藥材都有一種或 多種特殊活性化學物質或指標成份,例如三七的重要成份人參皂苷Rb1及Rg1,以 及三七皂苷R1,含量多寡影響該中藥材的效用。經由實驗所對以上雜質等進行化 驗,以及使用高壓液相層析儀(HPLC)及對應的標準品測定該成份的含量,再將所 有化驗結果對比標準限度,如《香港中藥材標準》內的規定限度,即可鑒定該中 藥材的品質。 三七樣品的指標成份含量 Assay of the Radix Notoginseng Sample
- 16. Contaminants Determination 污染物分析 Contaminant Limit (not more than) mg/kg Heavy Metals Arsenic砷, Cadmium鎘, Lead鉛, Mercury汞 2.0, 1.0, 5.0, 0.2 Pesticide Residues Aldrin and Dieldrin艾氏劑及狄氏劑 0.05 Chlordane氯丹 0.05 DDT滴滴涕 1.0 Endrin異狄氏劑 0.05 Heptachlor七氯 0.05 Hexachlorobenzene六氯苯 0.1 Hexachlorocyclohexane isomers六六六 0.3 Lindane林丹 0.6 Quintozene五氯硝基苯 1.0 Mycotoxins – Aflatoxin B1 & Aflatoxins黃曲霉毒素 5 &10 ug/kg
- 17. Benefit of the Certification Scheme 認證計劃的裨益
- 18. Why HK Needs a PCS for CMM? 為甚麼香港需要中藥材產品認證計劃? • Chinese medicine has become more popular worldwide 近年中藥發展快速,受到各國重視 • Supported by the National 12th Five-Year Plan to nurture the emerging industries , HK is privileged in view of its special position, technologies, experiences and credit to capture this opportunity through establishing itself as a Chinese medicine international trading center 中國「十二五」大力推進中醫藥科技繼承與創新,以及支持香港發展 新興產業。香港就其特殊位置、經驗、技術及信譽等,具發展成為中 藥國際貿易區潛力 • To develop the Chinese medicine trading, laboratory testing, and certification industries 推動本地的中藥業、測試和認證行業的發展 • In line with the development of emerging industries, and also enhancing consumers confidence. 配合香港對中醫中藥及新興產業的發展,亦可加強市民對中藥材的信 心
- 19. What is the Benefits of the PCS to CMM? 產品認證對中藥材供應商或製造商有何好處? • • • • • • • Promoting quality product quality through upstream control 產品認證過程會透過上遊監控提升產品質素 Create business opportunities when products being certified 已獲認證的產品能為公司創造商機 Through third-party involvement Increase the confidence to the products’ quality 通過第三方參與,增強使用者對產品質量的信心 Strengthen the quality management 強化中藥材的品質管理 Effective product promotion 有效推廣產品 Improve company image 提升企業形象 Reduce liability and recall of products 降低風險責任和退貨的機會
- 20. The Scheme Applicants 中藥材產品認證計劃的申證機構 • CM trades with effective license in HK 在香港從事中藥材業務,如中藥材製造、中藥材出口貿易、中藥材進 口、中藥材批發等,並持有有效及相關的香港牌照。 • To ensure the CMM quality conformity, the applicant should establish and operate a CMM quality operational system or procedure for daily operations, CMM procurement & sales, CMM storage & test, documentation & records, etc 申證企業應制定及實行有效的中藥材品質管理及操作程序,包括日常 運作及設備、中藥材採購及銷售、中藥材品質化驗及儲存、文件及紀 錄等等,以確保中藥材的品質。
- 21. When is the PCS Ready? How to apply? 何時接受認證申請?可向甚麼機構申請? The CMM-PCS has been drafted and is under pilot testing. Once the PCS is promulgated, applicants may approach the qualified certification bodies for details and application. 中藥材產品認證計劃已初步完成,並正進行試 運行及最後修編工作,將於適當時間推出,企 業或讀者可留意有關的信息。當中藥材產品認 證計劃正式推出後,申證企業可向合資格的認 證機構查詢認證詳情及申請認證。
- 22. Thank You! K L Tsang General Manager Hong Kong Productivity Council Environmental Management Division 香港生產力促進局 環境管理部 (Tel.: 2788-5628) kltsang@hkpc.org