Application of Statistical and mathematical equations in Chemistry Part 1
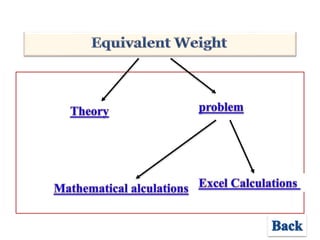
- 4. Problem – Equivalent Weight • Calculate the equiv. wt. (g/equiv.) • of ammonia NH3 ?
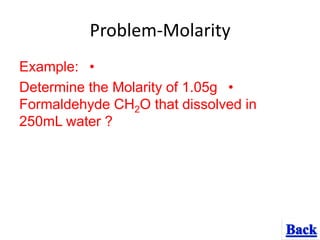
- 10. Problem-Molarity •Example: •Determine the Molarity of 1.05g Formaldehyde CH2O that dissolved in 250mL water ?
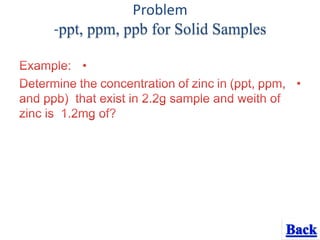
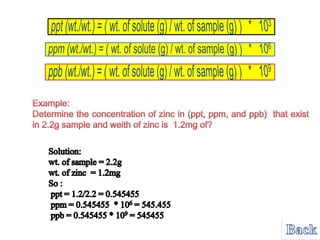
- 16. Problem -ppt, ppm, ppb for Solid Samples
- 22. Problem- Percent Concentration • Calculate the (%w/w), (%v/v), and (%w/v) for 3.0g of 10mL solute that exist in 100mL water ? (100mL = 100g of water).
- 23. Calculate the (%w/w), (%v/v), and (%w/v) for 3.0g of 10mL solute that exist in 100mL water ? (100mL = 100g of water). Solution : wt percent (%w/w) = 3g/100g = 3% vol percent (%v/v) = 10ml/100ml = 10% Wt.vol percent (%w/v) = 3g/100ml = 3% wt. percent (%w/w) = ( wt. of solute (g) / wt. of solution (g) ) * 100% vol. percent (%v/v) = ( vol. of solute (mL) / wt. of solution (mL) ) * 100% wt. vol. percent (%w/w) = ( wt. of solute (g) / vol. of solution (mL) ) * 100%
- 25. Problem- Density
- 26. Solution : 1.42g/cm3 = 1420g/L no of g. per liter =1420 *70 /100=994g/L M HNO3=499/63.0=16 mol./L