Atmospheric humidityfinal
- 1. TECHNOLOGICAL INSTITUTE OF THE PHILIPPINES938 AURORA BOULEVARD, CUBAO, QUEZON CITYCHAPTER IVATMOSPHERIC HUMIDITYDelivered and facilitated by: Glena Marie AngcaRonel G. BautistaRechelle R. ManaloEngr. Edison G. DizonInstructor
- 2. The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity.Nitrogen- 78.09%Oxygen- 20.95% Argon- 0.93% CO2- 0.039% Water vapor- 1%
- 3. Humidity- is the state of the atmosphere in relation to the amount of water vapor it contains.The amount of humidity found in air varies because of a number of factors. Two important factors are evaporation and condensation.
- 4. At the water, atmosphere interface over our planet's oceans, large amounts of liquid water are evaporated into atmospheric water vapor. This process is mainly caused by absorption of solar radiation and the subsequent generation of heat at the ocean's surface. In our atmosphere, water vapor is converted back into liquid form when air masses lose heat energy and cool. This process is responsible for the development of most clouds and also produces the rain that falls to the Earth's surface.
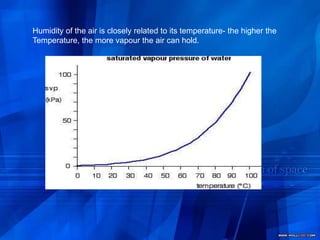
- 5. Humidity of the air is closely related to its temperature- the higher the Temperature, the more vapour the air can hold.
- 6. A Hygrometer is a device used for measuring the humidity of the air.
- 7. PROPERTIES OF VAPORVaporization - the change from the liquid to the gaseous state. Vaporization of liquid water occurs because some of its molecules possess sufficient kinetic energy to carry them out through the surface of the water in opposition to the attractive forces which tend to hold them within the body of the liquid.
- 8. Vapor pressure – In a mixture, each gas exerts a partial pressure independent of the other gases. The partial pressure exerted by water vapor is called vapor pressure.
- 9. Condensation – It is the process by which vapor changes to the liquid or solid state.
- 10. Latent Heat of Vaporization– The latent heat of vaporization is the amount of heat absorbed by a unit mass of a substance, without change in temperature, while passing from the liquid to the vapor state.HV = 594.9 – 0.15T where: HV - the latent heat in calories per gram of water T - temperature ( C)NOTE: latent heat refers to the amount of energy released or absorbed by a chemical substance during a change of state that occurs without changing its temperature.-a phase transition such as the melting of ice or the boiling of water
- 11. Latent Heat of Vaporization– The latent heat of vaporization is the amount of heat absorbed by a unit mass of a substance, without change in temperature, while passing from the liquid to the vapor state.HV = 594.9 – 0.15T where: HV - the latent heat in calories per gram of water T - temperature ( C)NOTE: latent heat refers to the amount of energy released or absorbed by a chemical substance during a change of state that occurs without changing its temperature.-a phase transition such as the melting of ice or the boiling of waterLatent Heat of Condensation– The latent heat of condensation is the amount of heat absorbed by a unit mass of a substance, without change in temperature, while passing from the vapor to liquidstate.
- 12. Latent Heat of Fusion – It is the amount of heat absorbed by a unit mass of substance, without change in temperature, while passing from a solid to a liquid.Latent Heat of Sublimation – It is the heat absorbed by a unit mass of substance , without change in temperature , while passing directly from a solid to a vapor state.
- 13. Density of Moist Air – The density of moist air is equal to the mass of water vapor plus the mass of dry air in a unit volume of the mixture. The density of air varies as the temperature and moisture content in the air varies. When the temperature increases the higher molecular motion results in an expansion of the volume and thus decreasing the density.ρ = 1 / vρ= (p / Ra T) (1 + x) / (1 + x Rw / Ra) where:v = specific volume of moist air per mass unit of dry air and water vapor (m3/kg)Ra = 286.9 – the individual gas constant air (J/kg K)Rw = 461.5 - the individual gas constant water vapor (J/kg K)x = specifi humidity or humidity ratio(kg/kg)p = pressure in the humid air (Pa)
- 14. SATURATED VAPOR PRESSUREThe evaporation of a liquidThe average energy of the particles in a liquid is governed by the temperature. The higher the temperature, the higher the average energy
- 16. The evaporation of a liquid in a closed containerAs the gaseous particles bounce around, some of them will hit the surface of the liquid again, and be trapped there. There will rapidly be an equilibrium set up in which the number of particles leaving the surface is exactly balanced by the number rejoining it.Particles continue to break away from the surface of the liquid - but this time they are trapped in the space above the liquid.
- 17. When these particles hit the walls of the container, they exert a pressure. This pressure is called the saturated vapour pressure (also known as saturation vapour pressure) of the liquid.
- 18. Measuring the saturated vapor pressureIf you squirt a few drops of liquid into the tube, it will rise to form a thin layer floating on top of the mercury. The saturated vapour pressure of the liquid will force the mercury level down a bit. You can measure the drop - and this gives a value for the saturated vapour pressure of the liquid at this temperature.at 1 atmosphere pressure the column will be 760 mm tall. 1 atmosphere is sometimes quoted as 760 mmHg ("millimetres of mercury").
- 19. HUMIDITY EXPRESSIONSVapor pressure- is the partial pressure of the water vapor at the atmosphere. It is usually expressed in millibars and can be computed by the empirical psychrometric equation.
- 20. Absolute humidity- is the mass of water vapor per unit volume of space. It is usually expressed in grams per cubic meter
- 21. Relative Humidity- is the percentage ratio of the amount of moisture in a given space to the amount which that volume could contain if it were saturated.
- 22. Dewpoint- is the temperature at which air becomes saturated when cooled under constant pressure and with constant water vapor content
- 23. Specific humidity- is the mass of water vapor per uint mass of moist air. Usually expressed in grams per kilogram of moist air.
- 24. Mixing ratio- is the mass of water vapor per unit mass of perfectly dry air in a humid mixture. It is usually expressed in grams per kilogram of dry air.
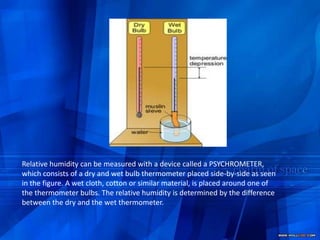
- 25. Precipitable water- the total amount of water vapor in the atmosphere is frequently expressed as the depth of precipitable water in inches.Relative humidity can be measured with a device called a PSYCHROMETER, which consists of a dry and wet bulb thermometer placed side-by-side as seen in the figure. A wet cloth, cotton or similar material, is placed around one of the thermometer bulbs. The relative humidity is determined by the difference between the dry and the wet thermometer.
- 27. INSTRUMENTS USED IN MEASURING ATMOSPHERIC HUMIDITYPsychrometer- generally used for making official measurements of humidity in the surface layers of the atmosphere. Hair Spectroscopic hygrometer – measures the selective absorption of light by water vapor in certainbands of the spectrum.Hygrometer – consist of a frame in which a strand of hair is kept at approximately constant tension.
- 28. Hair hygrograph – essentially a hair hygrometer, butthe hair activates a pen which records on a rotating drum.Hygrothermograph – combines the registration of bothrelative humidity and temperature on one record sheet.Clew point hygrometer – a device used to determine the dewpoint directly.ERRORS IN MEASUREMENTAll humidity – measuring instruments are subject to errors from improper observational technique but the ordinary psychrometer invites more errors because of the following reasons:First, two thermometers must be read
- 29. Second, sling and whirling psychrometers must be stopped to permit reading.
- 30. Third, latent heat of fusion causes the nercury in the wet-bulb thermometer to lag at the thermometer stem, insufficient ventilation, dirty or thick muslin, and impure water.HUMIDITY VARIATIONS WITH TIMEAnnual variation – The annual variation of the atmospheric – moisture content is, in most religions, practically parallel to that of temperature. Example is a summer maximum and a winter minimum.
- 31. Diurnal Variation - fluctuations that occur during each day.Sources:http://images.search.yahoo.com/images/view?back=http%3A%2F%2Fimages.search.yahoo.com%2Fsearch%2Fimages%3Fp%3Dhumidity%26ei%3DUTF-8%26fr%3Dslv8-msgr%26fr2%3Dtab-web&w=421&h=482&imgurl=eternalia.files.wordpress.com%2F2008%2F07%2Fhumidity.jpg&rurl=http%3A%2F%2Feternalia.wordpress.com%2F2008&size=102k&name=humidity+jpg&p=humidity&oid=c04713fd2cc48e96&fr2=tab-web&no=2&tt=854400&sigr=113a0lndh&sigi=11iafcr66&sigb=12prdl7v7http://images.search.yahoo.com/images/view?back=http%3A%2F%2Fimages.search.yahoo.com%2Fsearch%2Fimages%3Fp%3Datmosphere%26ei%3Dutf-8%26fr%3Dslv8-msgr&w=600&h=643&imgurl=www.tecnozono.com%2Fimages%2Fatmosphere.jpg&rurl=http%3A%2F%2Fwww.tecnozono.com%2Fenglish%2Fearth%27s_atmosphere.htm&size=88k&name=atmosphere+jpg&p=atmosphere&oid=588493cdb8e14782&fr2=&no=1&tt=3244597&sigr=11n1so26v&sigi=117f6onpr&sigb=12f62ef6hhttp://www.chemguide.co.uk/physical/phaseeqia/vapourpress.htmlhttp://hyperphysics.phy-astr.gsu.edu/Hbase/Kinetic/vappre.html