atom and atomic theory chemistryxxxx.ppt
- 1. Atoms and atomic theory of matter Jemimah Joy I. Guarin
- 2. Objectives: The students shall be able to: • State the atomic Theory of Matter • Describe the different atomic theories • Differentiate atoms from molecules
- 3. •Can you name an atomic theories of matter?
- 6. Atomic Theories of Matter • It seeks to explain the nature of matter- the materials of which the universe, all galaxies, solar systems and earth are formed. • First postulated by John Dalton, the atomic theory of matter contends: Each chemical element is made of fundamental units called atoms. • In 1808 an English scientist and school teacher, John Dalton, formulated a precise definition of the indivisible building blocks of matter that we call atoms. • Dalton’s work marked the beginning of the modern era of chemistry. • The hypotheses about the nature of matter on which Dalton’s atomic theory is based can be summarized as follows: 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass, and chemical properties. The atoms of one element are different from the atoms of all other elements
- 7. 3. Compounds are composed of atoms of more than one element. In any compound, the ratio of the numbers of atoms of any two of the elements present is either an integer or a simple fraction. 4. A chemical reaction involves only the separation, combination, or rearrangement of atoms; it does not result in their creation or destruction
- 8. Early concepts of the Atom • Around 400 BCE- Greek philosophers were debating whether matter was continuous or discrete (particulate). • Greek philosopher Democritus expressed the belief that all matter consist of very small, indivisible particles, which he named atomos (meaning uncuttable). • Aristotle- decided that matter was continuous and could be divided again and again. • “Billiard ball model” of atom by John Dalton- because he thought of atoms as essentially featureless, indivisible spheres of uniform density. • After 90 years, electron was discovered by J.J. Thomson at Cambridge University in England in 1897.
- 9. • Thomson studied electrical discharges in tubes of low-pressure gas called gas- discharge tubes or cathode-ray tubes. • He used a cathode ray tube and his knowledge of electromagnetic theory to determine the ratio of electric charge to the mass of an individual electron- -176 x 108 C/g, where C stands for coulomb. (9.10 x 10−28 g) • Thomson concluded that an atom was much like a sphere of plum pudding; electrons were the raisins stuck randomly in an otherwise homogeneous mass of positively charge pudding. (Thomson’s plum pudding model of atom)
- 10. • By the early 1900s, two features of atoms had become clear: they contain electrons, and they are electrically neutral. • In 1910, Ernest Rutherford was a physicist from New Zealand who studied at Cambridge University under Thomson. He devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. • During the experiment, most of the alpha particles did pass through the foil with little or no deflection. However, a very small number of the particles were deflected from their original paths at very large angles. • The only possible explanation was that the positive charge was not spread throughout the atom, but concentrated in a small, dense centre: the nucleus.
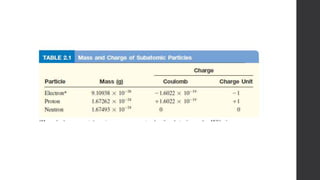
- 11. • In separate experiments, it was found out that proton has a mass of 1.67262 x 10−24 𝑔 . • Niels Bohr was a Danish physicist who set about trying to solve the problems with Rutherford’s model. He invoked quantum theory to try and explain the arrangement of electrons. • His model postulated the existence of energy levels or shells of electrons. • He proposed in 1913 a planetary model, where electrons revolve around the nucleus. • While the electrons are in orbit, they have “constant energy”, when these particles absorb energy and transition into a higher orbit they are “excited electrons”. When the electrons return to their original orbit, they give off this energy as electromagnetic radiation. • 1932, James Chadwick by his experiments discovered neutrons—electrically neutral particles having a mass slightly greater than that of protons. • The current atomic theory builds on work done in the 1930s by Albert Einstein, Werner Heisenberg and others. • Earlier theories, electrons and other tiny particles as definite solid “lumps” modern quantum theory treats them as a statistical “clouds”.
- 14. Atom • is the smallest possible piece of a chemical element. • All matter is made of groups of atoms bonded or connected, together into molecules. • The word atom comes from the Greek word “atomos”, which means “indivisible”.
- 15. Kinds of Atoms Stable Most atoms are stable. Their protons, neutrons and electrons balance. An atom that has enough binding energy to hold the nucleus together permanently. e.g. Noble gases Isotopes Atoms that have the same atomic number but different mass numbers. e.g. Hydrogen has three isotopes: hydrogen itself –has 1 proton and 0 neutron; deuterium—1 proton and 1 neutron; tritium—1 proton and 2 neutrons. Radioactive atoms have too many neutrons in the nucleus, which makes them unstable. They’re radioactive, giving off particles until they become stable. e.g. Uranium-238 and Uranium-235 can decay to form secondary radionuclides of radium and polonium.
- 16. Ions atoms with extra or missing electrons. They have a positive or negative electric charge and are responsible for many chemical reactions. e.g. 𝑁𝑎+ , 𝑀𝑔2+ Antimatter every atomic particle has a twin anti-particle, with an opposite electric charge. e.g. positron- the antiparticle of electron; antiproton- the antiparticle of the proton. Molecule an aggregate of at least 2 atoms in a definite arrangement held together by chemical forces or chemical bonds. “Kinds of Molecules” Monoatomic- made of only one atom. e.g. He Diatomic- containing two atoms. e.g. 𝐻2, 𝑂2 Polyatomic- containing more than two atoms. e.g. 𝐻2𝑂
- 20. Atomic Number & Isotopes Atomic Number- is the number of protons in the nucleus of each atom of an element. Mass Number- is the total number of neutrons and protons present in the nucleus of an atom of an element. Example: Boron: mass number= 11; atomic number= 5 (means 5 protons in the nucleus) to get the number of neutrons : 11 – 5 = 6
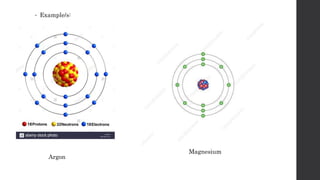
- 21. Isotopes -atoms that have the same atomic number but different mass numbers. Example/s:
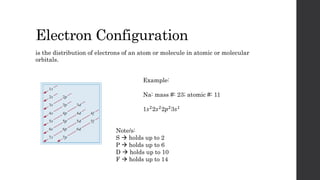
- 22. Electron Configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Example: Na: mass #: 23; atomic #: 11 1𝑠22𝑠22𝑝23𝑠1 Note/s: S holds up to 2 P holds up to 6 D holds up to 10 F holds up to 14
- 23. • Example/s: Mg 1s2 2s2 2p6 3s2 Cr 1s2 2s2 2p6 3s2 3p6 4s2 3d4 C 1s2 2s2 2p2 Practice! F 1s2 2s2 2p5 As 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 Si 1s2 2s2 2p6 3s2 3p2 Cu 1s2 2s2 2p6 3s2 3p6 4s2 3d9
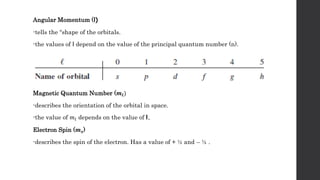
- 24. Quantum Numbers • To completely describe the distribution of electrons in an atom. Principal Quantum Number / energy (n) -can have integral values 1,2,3 etc…. -it relates to the average distance of the electron from the nucleus in a particular orbital. -the larger the n is, the greater the average distance of an electron in the orbital from the nucleus and therefore, the larger the orbital.
- 25. Angular Momentum (l) -tells the “shape of the orbitals. -the values of l depend on the value of the principal quantum number (n). Magnetic Quantum Number (𝒎𝒍) -describes the orientation of the orbital in space. -the value of 𝑚𝑙 depends on the value of l. Electron Spin (𝒎𝒔) -describes the spin of the electron. Has a value of + ½ and – ½ .
- 27. Groups in Periodic Table
- 28. Some element groups have given special names, such as: Group 1A elements alkali metals Group 2A elements alkaline earth metals Group 6A elements chalcogens Group 7A elements halogens Group 8A elements noble gases
- 29. Periodic Trends Atomic Radii -is ½ the distance between the two nuclei in two adjacent metal atoms or in a diatomic molecule. Ionic Radius -is the radius of a cation or an anion. Ionization Energy -is the minimum energy (in kJ/mol) required to remove an electron from a gaseous atom in its ground state. Electron Affinity -is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion.
- 30. Electronegativity -is a measure of the tendency of an atom to attract a bonding pair of electrons. Metallic Character -it refers to the level of reactivity of a metal. Chemical Reactivity -refers to how likely or vigorously an atom is to react with other substances.
- 32. Practice: Arrange the ff. in increasing of atomic radii: Sc, K, Ni, Ge, Kr Kr, Ge, Ni, Sc, K Arrange the ff. in increasing of Ionization Energy: Sc, K, Ni, Ge, Kr K, Sc, Ni, Ge, Kr Arrange the ff. in increasing of Electronegativity: Sc, K, Ni. Ge, Kr K, Sc, Ni, Ge, Kr