Atomic structure
- 1. STRUCTURE OF ATOM Made by :Dr. Isha jaiswal Moderator :Dr. S.P.MISHRA
- 3. •Changes in view of “aToM”
- 4. •Democritus (400 B.C.) • Proposed that matter was composed of tiny indivisible particles • Philospher; Not based on experimental data. • Greek: atomos
- 5. •John Dalton (1807) • British Schoolteacher • Dalton’s postulate • Proposed Ball Model • atom is a uniform, solid sphere
- 6. •J. J. Thomson • Cathode Ray Tube Experiments • beam of negative particles • Discovered Electrons • negative particles within the atom • Plum-pudding Model
- 7. •Thomson’s Cathode Ray Experiment Stream of electrons is attracted to positively charged plate here. "
- 8. •J. J. Thomson Plum-pudding Model • positive sphere (pudding) with negative electrons (plums) dispersed throughout
- 9. •Ernest Rutherford Student of J.J THOMSON Nobel prize winner. Rutherford performed the famous ‘GOLD FOIL EXPERIMENT’. Discovered NUCLEUS :dense, positive charge in the center of the atom. Proposed NUCLEAR MODEL
- 11. •Gold Foil Experiment Cont.
- 12. • Nuclear Model • dense, positive nucleus surrounded by negative electrons
- 13. •James Chadwick (1932) • Discovered neutrons • neutral particles in the nucleus of an atom
- 14. •James Chadwick (1932) Neutron Model • revision of Rutherford’s Nuclear Model
- 15. •Niels Bohr • Father of QUANTUM PHYSICS • Revised RUTHERFORD’S model .eletron is accelerating charge. • Energy Levels • electrons can only exist in specific energy states
- 16. • Bright-Line Spectrum : • tried to explain presence of specific colors in hydrogen’s spectrum • Planetary Model • electrons move in circular orbits within specific energy levels Bright-line spectrum
- 17. •Erwin Schrödinger : Electron Cloud Model (orbital) • dots represent probability of finding an e- not actual electrons
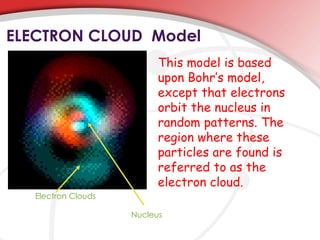
- 18. ELECTRON CLOUD Model This model is based upon Bohr’s model, except that electrons orbit the nucleus in random patterns. The region where these particles are found is referred to as the electron cloud. Electron Clouds Nucleus
- 19. • The Nucleus and Structure of the Atom • Atoms are made of three kinds of particles: electrons, protons, and neutrons.
- 20. • The structure of the atom • The protons & neutrons are present in nucleus • Electrons are outside the nucleus in the electron cloud.
- 21. • The representation of atom
- 22. Nomenclature for Elements "X" = Element Symbol "Z" = ATOMIC NUMBER: no.of Protons Each eleMenT has a unique "Z” "N” = no.of Neutrons "A" = ATOMIC MASS:no.of neutron +proton ( A = Z + N) ISOTOPE: Atoms of same elements with same atomic no. but different mass no. Isobar: ATOMS OF DIFFERENT ELEMENT HAVING SAME MASS NO. BUT DIFFERENT ATOMIC NO. X A Z
- 23. ATOMIC &NUCLEAR STABILITY: the forces of NATURE.
- 24. • The strong nuclear force attracts neutrons and protons to each other, otherwise the positively charged protons would repel each other.
- 25. • Electrons are bound to the nucleus by electromagnetic forces. • The force is the attraction between protons (positive) and electrons (negative). The momentum of the electron causes it to move around the nucleus rather than falling straight in.
- 26. 26 • As a general rule, a nucleus will need a neutron/proton ratio of 3:2 (or 1.5:1) in order to stay together.
- 27. 27 As atomic mass increases, the neutron to proton ratio for stable nuclei increases because proton-proton repulsion becomes significant!!! Nuclear forces arise form neutrons, so the neutron to proton ratio must increase for heavier elements. Belt of Stability Proton number, Z Neutronnumber,N=A-Z N = Z For helium He- 4 the N:P ratio is 1 : 1 For uranium U- 238 the ratio is 1 : 1.6
- 28. • If an isotope has too many (or too few) neutrons, the nucleus eventually breaks up and we say the atom is radioactive. • In a stable isotope the nucleus stays together.
- 29. 29 • The amount of energy that keeps a nucleus together is called the Binding Energy. • This amount of energy is higher for nuclei that are stable than it would be for unstable nuclei.
- 30. 30 Mass Defect The difference between the mass of the atom and the sum of the masses of its parts is called the mass defect (Dm). measurements show that the mass of a particular atom is always slightly less than the sum of the masses of the individual protons, neutrons and electrons of which the atom consists. e.g. a helium nucleus consists of 2 protons and 2 neutrons 2 protons & 2 neutrons Helium atom
- 31. 31 Mass defect can be converted into equivalent energy called as binding energy. Using Einstein’s E = mc2, this is equivalent to a loss of energy This figure is the BINDING ENERGY . THE BINDING ENERGY of a nucleus is defined as the energy which must be input to separate all of its protons and neutrons.
- 32. 32 Binding Energies are usually expressed in MeV 1 amu = 931.3 MeV To compare the stabilities of different nuclei, Binding Energies PER NUCLEON in the nucleus are compared. The higher the binding energy per nucleon, the greater the stability of the nucleus
- 33. 33 NUCLEON NUMBER BINDING ENERGY Per NUCLEON 2H 238U 56Fe 8.8 MeV Iron is the most stable nucleus
- 34. 34 FISSION IRON Heavy nuclei may increase their stability by Nuclear Fission Light nuclei may increase their stability by Nuclear Fusion FUSION
- 35. 35 Nuclear Fission is the fragmentation of heavy nuclei to form lighter, more stable ones. The Fission of U - 235 U235 92 energyofMeVnKrBanU 18031 0 94 36 139 56 1 0 235 92 This is only one of several fissures that are possible. On average 2.5 neutrons are released
- 36. 36 Critical mass :is the mass required for the chain reaction to become self-sustaining. neutron Some neutrons may : Cause more fission Get lost Be absorbed by an atom lost For a chain reaction to be self sustaining, every fission must produce at least one more neutron that will initiate further fissions.
- 37. Every good conversation starts with good listening. Thankyou verymuch for ur patient listening. Special thanxs to S.PMISHRA SIR for his guidance and support.