Beckmann Rearrangement Y.K.pptx
- 1. BECKMANN REARRANGEMENT PRESENTED BY: YEHTESHAM KHURSHID M.PHARM PHARMACEUTICAL CHEMISTRY FIRST SEMESTER SPER, JAMIA HAMDARD
- 2. CONTENTS 1.INTRODUCTION 2.MIGRITORY APTITUDE 3.GENERAL REACTION 4.EXAMPLE OF REACTION 5.MECHANISM OF REACTION 6.APPLICATION 7.REFERENCES
- 3. INTRODUCTION ✔ The Beckmann rearrangement reaction is one of the most employed reaction in many sector to convert oximes to amides. This reaction is discovered by the chemist Ernst otto Beckmann in the mid-1880. ✔ The Beckmann rearrangement is the acid catalyzed conversion of oximes into their corresponding amides that allows the insertion of nitrogen atom from the C=N double bond into the carbon chain forming a C-N single bond. ✔ The reaction involves migration of group from carbon to nitrogen. ✔ Concentrated Sulphuric acid , Hydrochloric acid, Phosphorus pentachloride, Phosphorus tetrachloride, Sulphonyl chloride and Zinc oxide are generally used as reagent.
- 4. MIGRATORY APTITUDE ✔ The migration of group depends not on the migration aptitude but upon the orientation of the group in relation to –OH group. It is found that the migration group is always anti to –OH group and thus the rearrangement is said to be stereospecific. ✔ When chiral groups are present ,they migrate intramolecularly with retention of configuration. ✔ Migratory aptitude- Phenyl > Ethyl> Methyl
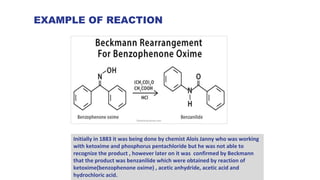
- 6. EXAMPLE OF REACTION Initially in 1883 it was being done by chemist Alois Janny who was working with ketoxime and phosphorus pentachloride but he was not able to recognize the product , however later on it was confirmed by Beckmann that the product was benzanilide which were obtained by reaction of ketoxime(benzophenone oxime) , acetic anhydride, acetic acid and hydrochloric acid.
- 7. Caprolactam was discovered by Otto Wallach in 1900 and its commercial synthesis of acid catalysed Beckmann rearrangement of Cyclohexanone oxime.
- 8. MECHANISM OF REACTION ⮚ Protonation of oxime • The protonation at the oxygen atom of oxime gives oxonium cation. ⮚ Migration of alkyl group • The alkyl group transfer to nitrogen atom followed by removal of water molecule that lead to formation nitrilium cation. ⮚ Hydrolysis of nitrilium cation • The nitrilium cation get hydrolysed to give an amide as the final product in the presence of basic medium .
- 9. oxime Amide
- 10. APPLICATION ● Synthesis of isoquinoline:
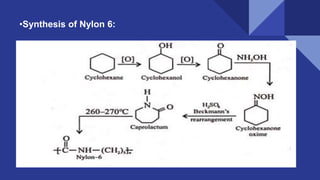
- 11. •Synthesis of Nylon 6:
- 12. ● Synthesis of Lactams: Synthesis of lactams Alicyclic ketones of all ring sizes undergo the Beckmann rearrangement of their oximes to yield lactams. A product of considerable industrial importance is perlon (valuable textile polymer) which is prepared from w-caprolactam. This is obtained by the Beckmann rearrangement of cyclohexanone oxime It is synthesized from phenol.
- 13. References ✔ Morrison , R. T &Boyed, R.N, organic chemistry ✔ March , J, advanced organic chemistry, Wiley eastern limited ✔https://chemistnotes.com ✔ https://www.organic-chemistry.org ✔ https://www.chemistrylearner.com/beckmann-rearrangement. ✔ P.Yan , P. Batamack, G.K.S Prakash and G.A Olah,Catal.Lett,2005, page103 and 165-168. ✔ S. Srivastava and K. Kaur, New J. Chem, 2020, page no-3
- 14. THANK YOU