Bhor's Atomic model
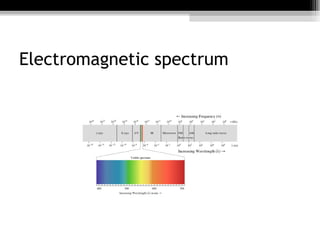
- 1. ELECTROMAGNETIC RADIATION The general term for the entire range of energy that travels through space as waves and at the speed of light. Is classified into several types according to the frequency of its wave; these types include (in order of increasing frequency and decreasing wavelength): radio waves , microwaves , terahertz radiation , infrared radiation , visible light , ultraviolet radiation , X-rays and gamma rays .
- 2. Wavelength ( λ ) : The length of one wave, or the distance from a point on one wave to the same point on the next wave. Frequency of oscillation ( f ) (or just frequency ): the number of times the wave pattern repeats itself in one second.
- 3. r
- 6. Classic mechanics: is a set of principles describing objects that move at ordinary velocity and are macroscopic. Quantum mechanics (QM) is a set of principles describing physical reality at the atomic level of matter ( molecules and atoms ) and the subatomic ( electrons , protons , and even smaller particles ). Studies particles that moves at the speed of light (3x10 8 m/s)
- 7. The Bohr Model Is known as the "planetary model" of the atom that is used as a symbol for atomic energy levels. the neutrons and protons are in the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun . Second energy level First energy level
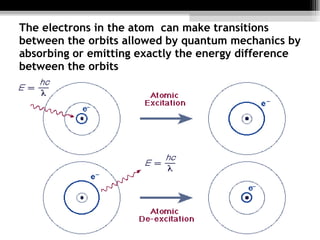
- 9. The Bohr model consists of these principles Electrons assume only certain orbits around the nucleus. These orbits are stable. Each orbit has an energy associated with it. For example the orbit closest to the nucleus has an energy E1, the next closest E2 and so on. Light is emitted when an electron jumps from a higher orbit to a lower orbit and absorbed when it jumps from a lower to higher orbit.
- 10. The basic feature of quantum mechanics that is incorporated in the Bohr Model in the Bohr atom is restricted to certain discrete values. One says that the energy is quantized . This means that only certain orbits with certain radii are allowed; orbits in between simply don't exist. The lowest energy state is generally termed the ground state . The states with more energy than the ground state are called the excited state.
- 11. The electrons in the atom can make transitions between the orbits allowed by quantum mechanics by absorbing or emitting exactly the energy difference between the orbits
- 12. Bohr's model was so successful that he immediately received world-wide fame. Unfortunately, Bohr's model worked only for hydrogen. Thus the final atomic model was yet to be developed.
- 13. Quantum Numbers The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom. It therefore required four coordinates, or four quantum numbers , to describe the orbitals in which electrons can be found. the principal ( n ), angular ( l ), magnetic ( m ) and spin (s)quantum numbers. These quantum numbers describe the size, shape, orientation in space and spin of the orbitals on an atom.
- 14. The principal quantum number ( n ) describes the size of the orbital. Orbitals for which n = 2 are larger than those for which n = 1, for example. The principal quantum number therefore indirectly describes the energy of an orbital. The angular quantum number ( l ) describes the shape of the orbital. Orbitals have shapes that are best described as spherical ( s ), two lobes ( p ), or complex (d and f). The magnetic quantum number (m) describes the projection of the orbital angular momentum along a specified axis (x, y or z). The spin quantum number (s) describes the spin of the electron (-1/2 = counter-clockwise, 1/2 = clockwise).
- 15. Quantum number l
- 16. Quantum number m
- 17. Orbital : shows only the regions around the nucleus in which an electron has a relatively high probability of being found. Pauli exclusion principle : States that no more than two electrons (each with opposite spin ) can coexist in a single orbital. Hund’s Rule : States that for the electrons in the same energy level have the tendency to occupy empty orbital avoiding overlapping with other electron. The Aufbau Procedure The aufbau procedure (filling order of atomic orbitals) is used to work out the electron confiturations of all atoms.
- 18. Hund’s diagonal Rule s 2 p 6 d 10 f 14
- 21. Example Sulfur Z=16 this means 16 electrons Use diagonal rule Electron’s configuration diagram 1s 2s 2p 3s 3p Electron’s configuration 1s 2 2s 2 2p 6 3s 2 3p 4 *Check periodic table the energy level 3 is the period 3 And the position p the fourth place is sulfur
- 22. S
- 24. Draw the electron configuration diagrams of the elements of the period 2 Write the electron configuration of the elements from period 3 Li Be B C N O F Ne Na Mg Al Si P S Cl Ar