Blotting techniques
- 1. BLOTTING TECHNIQUES DIPESH TAMRAKAR M.Sc. Clinical Biochemistry 1
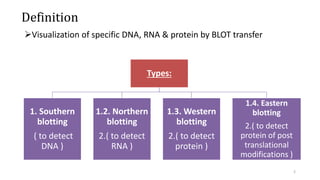
- 2. Definition Visualization of specific DNA, RNA & protein by BLOT transfer Types: 1. Southern blotting ( to detect DNA ) 1.2. Northern blotting 2.( to detect RNA ) 1.3. Western blotting 2.( to detect protein ) 1.4. Eastern blotting 2.( to detect protein of post translational modifications ) 2
- 3. 1. Southern Blotting The technique was developed by E.M. Southern in 1975 Used to detect the presence of a particular fragment of DNA in a sample This DNA can be: • Single gene • Part of a larger piece of DNA s/a viral genome Southern blot; a method for transferring DNA from an agarose gel to nitrocellulose filter , on which the DNA can be detected by suitable probe ( eg : complementary DNA or RNA ) 3
- 4. “Used to detect the DNA” • This method Involves: 1. Separation 2. Transfer 3. Hybridization. “The key to this method is Hybridization” 4
- 5. DNA Each individuals unique genetic blueprint is stored in material known as DNA. DNA is found in all cells containing a nucleus. DNA can be extracted for analysis from hair, bones, saliva, sperm, skin, organs, all body tissues and blood. Temperature Storage for DNA Purified DNA may be refrigerated at 4°C for up to 3 years. Samples kept over 3 years should be frozen at -70°C. • Specimens used in DNA testing: • Whole blood, Solid tissue, Serum and plasma • Urine, Bone marrow and many others 5
- 6. 1. Separation The DNA sample is digested by restriction endonucleases , producing small fragments & that are amenable for analysis . Fragments are separated by agarose gel electrophoresis or PAGE . The mobility of nucleic acids in agarose gels is influenced by agarose concentration , molecular size & molecular conformation of the nucleic acid . Agarose concentration of 0.3 – 2% are most effective for nucleic acid separation . Like proteins, nucleic acids migrate at rate that is inversely proportional to the logarithm of their molecular weight . 6
- 7. Separated nucleic acids are visualized by fluorescent dye ethidium bromide The agarose gel is soaked in a solution of dye & washed for remaining excess dye illumination of the rinsed slab with UV light reveals red orange stains where nucleic acids are located Ethidium bromide stains both single & double stranded nucleic acids , the fluorescence is much greater with double stranded molecules The electrophoresis can be performed with dye incorporated in the gel & buffer This has the advantage that the gel can be illuminated with UV light during electrophoresis to view the extent of separation. 7
- 8. The mobility of DNA may be reduced by 10 -15 % in the presence of ethidium bromide Ethidium bromide must be used with great care as it is a potent mutagen Newer fluorescent SYBR dyes produced by molecular probes offer several advantages , less toxic & 5 times more sensitive than ethidium bromide Labeled DNA with radioisotope P32 at 5’ & 3’ ends P32 is a strong β emitter Bands of labeled DNA on electrophoresis gel can located by autoradiography 8
- 9. • Labelling molecule before analysis with coenzyme biotin , biotin forms a strong complex with enzyme linked streptavidin . • PAGE is useful for analyzing small fragments of DNA upto 500 bp • Large molecules of DNA could be separated by pulsed field gel electrophoresis. 9
- 10. • Ethidium bromide is an intercalating agent commonly used as a fluorescent tag • It is commonly abbreviated as "EtBr", which is also an abbreviation for bromoethane • When exposed to ultraviolet light (210 nm), it will fluoresce with an orange colour, intensifying almost 20-fold after binding to DNA • EtBr emits orange light with wavelength 605 nm. Ethidium bromide intercalated between two adenine-thymine base pairs. 10
- 11. 2. Blotting • Transfer of DNA from gel to nitrocellulose membrane done by 1 ) Weak acid treatment to depurinate & fragment the DNA , thus make it smaller & easier to elute from the gel . followed by 2) Denaturate with strong base & neutralization ( hydrolyzes phosphodiester back bone at depurinated sites ) • single strands bind to membranes more efficiently • A buffer is used to facilitate the transfer . 11
- 12. a. Capillary blotting: The gel is placed on a wet pad of buffer- soaked filter paper & sheet of nitrocellulose placed on the gel Buffer is then drawn through the gel by placing a pad of dry absorbent material (usually filter paper) followed by a heavy weight on top of the sheet Passage of buffer by capillary action through the gel carries the separated nucleic acids on the nitrocellulose sheet to which they bind irreversibly by hydrophobic interaction 12
- 13. b. Electro blotting: Quicker & more efficient method of transfer Sandwich of gel & nitrocellulose compressed in a cassette & immersed in buffer between two parallel electrodes A current is passed at right angles to the gel which causes the separated nucleic acids to electrophorese out of the gel & into nitrocellulose sheet Obtained blot can be analyzed 13
- 14. 3. Hybridization The key to this method is hybridization. Hybridization-process of forming a double-stranded DNA molecule between a single-stranded DNA probe and a single-stranded target patient DNA. There are 2 important features of hybridization: The reactions are specific-the probes will only bind to targets with a complementary sequence. The probe can find one molecule of target in a mixture of millions of related but non-complementary molecules. 14
- 15. • Steps for hybridization: 1. The mixture of molecules are separated. 2. The molecules are immobilized on a matrix. 3. The probe is added to the matrix to bind to the molecules. 4. Any unbound probes are then removed. 5. The place where the probe is connected corresponds to the location of the immobilized target molecule detected by autoradiogarphy 15
- 16. Probe labelling & detection: 1. Radioactively labeled, often with 32P because of its high energy and ease of incorporation into the phosphate groups of dNTPs & detected using X-ray film. 2. Colorimetric detection (colored complex); a. Alkaline Phosphatase that acts on substrates 5-bromo-4-chloro- indoylphosphate (BCIP) to produce a dark purple product) b. Horse Reddish Peroxidase (HRP) acts on H2O2 to produce insoluble brown product c. HRP acts on 4-chloro-1-napthol into insoluble blue product 3. Fluorescent detection 4. Chemiluminescence detection (luminol, isoluminol) 16
- 17. Applications Southern blots are used in gene discovery, mapping , evolution & development studies , diagnostics & forensics . Allow for determination of molecular weights of restriction fragments Presence of particular bit of DNA in the sample. Identify: mutations, deletions, insertions and gene rearrangements Used in prognosis of cancer and in prenatal diagnosis of genetic diseases Leukemias Diagnosis of HIV-1 and infectious disease Applications of DNA fingerprinting include: • Paternity and Maternity Testing • Criminal Identification and Forensics • Personal Identification 17
- 18. Steps in southern blotting The DNA is digested Fragments Gel electrophoresis Transfer to membrane Probing Autoradiogram 18
- 19. 19
- 20. Northern blotting • Northern blotting is a technique for detection of specific RNA sequences . • Developed by James Alwine & George stark. • RNA molecules have defined length & much shorter than genomic DNA it is not necessary to cleave RNA before electrophoresis . • RNA is more susceptible to degradation than DNA . • RNA sample are separated based on size by gel electrophoresis • Northern blotting was developed 20
- 21. • RNA is blotted on to a nylon positively charged membrane . • The membrane is placed in a hybridization buffer with a labeled probe ( usually DNA ) • Labeled probe is detected by autoradiography • Expression patterns of sequences of interest in different samples can be compared . Northern blotting 21
- 22. Applications • A standard for the direct study of gene expression at the level of mRNA (mRNA transcripts) • Detection of mRNA transcript size • Study RNA degradation • Study RNA splicing - can detect alternatively spliced transcripts • Study RNA half-life • Study IRES (Internal Ribosomal Entry Site) – to remove possibility of RNA digestion vs. 2nd cistron translation. Northern blotting 22
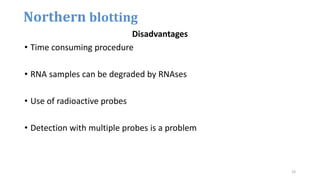
- 23. Disadvantages • Time consuming procedure • RNA samples can be degraded by RNAses • Use of radioactive probes • Detection with multiple probes is a problem Northern blotting 23
- 24. Steps involved in Northern Blotting RNA isolation Loading of sample on Agarose gel Blotting on nitrocellulose membrane Labeling with probe Washing to remove unbound probe Detection by autoradiogram 24
- 25. Western blotting • Dr. Douglas Lake of the University of Arizona School of Medicine's Department of Microbiology and Immunology • Western blotting is an immunoblotting technique which rely on the specificity of binding between the molecule of interest & a probe to allow detection of molecule of interest in a mixture of many other similar molecules . • In western blotting the molecule of interest is a protein & the probe is typically an antibody raised against that particular protein . 25
- 26. 26
- 27. • SDS PAGE technique is a prerequisite for western blotting • Protein sample is subjected to electrophoresis on SDS polyacrylamide gel • Electroblotting transfers the separated proteins from the gel to the surface of nitrocellulose membrane • Blot is incubated with primary and secondary antibodies on the nitrocellulose • Detection by autoradiography or fluorescence or colorimetric or chemiluminescence detection Western blotting 27
- 28. Blocking • Used to prevent the interactions between the membrane and the antibody used for detection of the target protein. • Blocking of non-specific binding is achieved by placing the membrane in a dilute solution of protein – typically 3–5% bovine serum albumin (BSA) or non-fat dry milk in tris-buffered saline (TBS), with a minute percentage (0.1%) of detergent such as Tween 20 or Triton X-100. • The protein in the dilute solution attaches to the membrane in all places where the target proteins have not attached. • Thus, when the antibody is added, there is no room on the membrane for it to attach other than on the binding sites of the specific target protein. • This reduces background in the final product of the western blot, leading to clearer results, and eliminates false positives. Western blotting 28
- 29. Tissue preparation • Samples can be taken from whole tissue or from cell culture. • Solid tissues are first broken down mechanically using a blender using a homogenizer or by sonication. • Assorted detergents, salts, and buffers may be employed to encourage lysis of cells and to solubilize proteins. • Protease and phosphatase inhibitors are often added to prevent the digestion of the sample by its own enzymes. • A combination of biochemical and mechanical techniques – comprising various types of filtration and centrifugation – can be used to separate different cell compartments and organelles. Western blotting 29
- 30. • An antibody which is specific for the protein of interest ( the primary antibody Ab 1 ) is added to the nitrocellulose sheet & reacts with the antigen • Only the band containing protein of interest binds the antibody forming a layer of antibody molecules • Following several rinses for removal of nonspecifically bound Ab1 , the Ab1 – antigen complex on the nitrocellulose sheet is incubated with second antibody Ab2 , which specifically recognizes the Fc domain of the primary antibody & binds it . • Ab 2 is radioactive labeled or is covalently linked to reporter enzyme which allows to visualize protein – Ab1 – Ab2 complex . Western blotting 30
- 31. Applications • The confirmatory HIV test employs a western blot to detect anti HIV antibody in a human sample . • Proteins from known HIV infected cells are separated & blotted on a membrane then the serum to be tested is applied in the primary antibody incubation step. • Free antibody is washed away & a second anti human antibody linked to an enzyme signal can be added . Western blotting 31
- 32. • The stained bands then indicate the proteins to which the patient serum contains antibody • Western blot is also used as definitive test for bovine spongiform encephalopathy ( mad cow disease ) • Some forms of Lyme disease testing employs western blotting • Confirmatory test for Hepatitis B infection and HSV-2 infection Western blotting 32
- 33. Western blotting in Biochemistry study different properties of protein based on molecular weight Helps in confirm effective inhibition of myocardial mTOR kinase activities by analyzing protein molecular wt. Vs expected values investigations of mitochondrial uniporter selective calcium channels in the organelle's inner membrane: Using the western blot technique, researchers were able to determine through phylogenetic analysis that the uniporter must have been a feature of the earliest mitochondria. Also used in Blood Doping to detect drug abuse in sports 33
- 34. 34
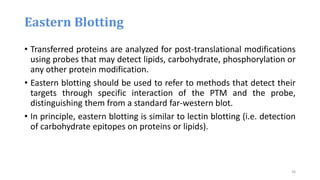
- 35. Eastern Blotting • developed by Towbin in 1979 • The eastern blot is a biochemical technique used to analyze protein post translational modifications (PTM) such as lipids, phosphomoieties and glycoconjugates. • It is most often used to detect carbohydrate epitopes. • Thus, eastern blotting can be considered an extension of the biochemical technique of western blotting. • Multiple techniques have been described by the term eastern blotting, most use proteins blotted from SDS-PAGE gel on to a nitrocellulose membrane. 35
- 36. • Transferred proteins are analyzed for post-translational modifications using probes that may detect lipids, carbohydrate, phosphorylation or any other protein modification. • Eastern blotting should be used to refer to methods that detect their targets through specific interaction of the PTM and the probe, distinguishing them from a standard far-western blot. • In principle, eastern blotting is similar to lectin blotting (i.e. detection of carbohydrate epitopes on proteins or lipids). Eastern Blotting 36
- 37. Application • detection of protein modifications in bacterial species Ehrlichia- E. muris and IOE. • The technique showed that the antigenic proteins of the non-virulent E. muris is more post-translationally modified than the highly virulent IOE • PMT play an important role in translocation across biological membranes. • Expression of post-translated proteins is important in several diseases Eastern Blotting 37
- 38. • Far-eastern blotting is a technique developed in 1994 by Taki and colleagues at the Tokyo Medical and Dental University, Japan for the analysis of lipids separated by high-performance thin layer chromatography (HPTLC). • The lipids are transferred from the HPTLC plate to a polyvinyledene difluoride (PVDF) membrane for further analysis, for example by enzymatic or ligand binding assays or mass spectrometry. Far-Eastern blotting 38
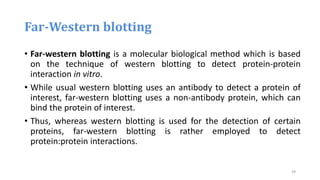
- 39. Far-Western blotting • Far-western blotting is a molecular biological method which is based on the technique of western blotting to detect protein-protein interaction in vitro. • While usual western blotting uses an antibody to detect a protein of interest, far-western blotting uses a non-antibody protein, which can bind the protein of interest. • Thus, whereas western blotting is used for the detection of certain proteins, far-western blotting is rather employed to detect protein:protein interactions. 39
- 40. Northwestern blot • The Northwestern blot, also known as the Northwestern assay, is a hybrid analytical technique of the Western blot and the Northern blot, • used in molecular biology to detect interactions between RNA and proteins. • The Northwestern blot combines the two techniques, and specifically involves the identification of labeled RNA that interact with proteins that are immobilized on a similar nitrocellulose membrane. 40
- 41. 41