Bomb calorimetry
- 1. Bomb Calorimetry Dr. K. Shahzad Baig Memorial University of Newfoundland (MUN) Canada Petrucci, et al. 2011. General Chemistry: Principles and Modern Applications. Pearson Canada Inc., Toronto, Ontario. Tro, N.J. 2010. Principles of Chemistry. : A molecular approach. Pearson Education, Inc.
- 2. Heats of Reaction and Calorimetry A chemical reaction is a process in which some chemical bonds are broken and others are formed, in general, the chemical energy of a system is changed as a result of a reaction. A heat of reaction, is the quantity of heat exchanged between a system and its surroundings when a chemical reaction occurs within the system at constant temperature. One of the most common reactions studied is the combustion reaction. This is such a common reaction that we often refer to the heat of combustion when describing the heat released by a combustion reaction
- 3. An endothermic reaction, is the one a temperature decrease in an isolated system or a gain of heat from the surroundings by a nonisolated system, heat of reaction is a qrxn > 0 An endothermic reaction Ba (OH)2 . 8H2O (s) and NH4Cl (s) and are mixed at room temperature, and the temperature falls to 5.8 oC in the reaction. An exothermic reaction is one that produces a temperature increase in an isolated system or, gives off heat to the surroundings, in a non-isolated system, the heat of reaction is (qrxn < 0) An exothermic reaction. Slaked lime, Ca (OH)2 is produced by the action of H2O on quicklime, (CaO). The reactants are mixed at room temperature, but the temperature of the mixture rises to 40.5 oC 𝐶𝑎𝑂 𝑠 + 𝐻2 𝑂 𝑙 → 𝐶𝑎 𝑂𝐻 2 𝐵𝑎 (𝑂𝐻)2 . 8𝐻2 𝑂 𝑠 + 2𝑁𝐻4 𝐶𝑙 𝑠 → 𝐵𝑎𝐶𝑙2 2 𝐻2 𝑂 𝑠 + 2𝑁𝐻3 𝑎𝑞 + 8𝐻2 𝑂 𝑙
- 4. Bomb Calorimetry Used to measure the heat evolved in a combustion reaction. The system is everything within the double- walled outer jacket of the calorimeter. This includes the bomb and its contents, the water in which the bomb is immersed, the thermometer, the stirrer, and so on. The system is isolated from its surroundings. . 𝒒 𝒓𝒙𝒏 = − 𝒒 𝒄𝒂𝒍𝒐𝒓𝒊𝒎 𝒘𝒉𝒆𝒓𝒆 𝒒 𝒄𝒂𝒍𝒐𝒓𝒊𝒖𝒎 = 𝒒 𝒃𝒐𝒎𝒃 + 𝒒 𝒘𝒂𝒕𝒆𝒓 … qcalorim = heat capacity of calorium * ∆T When the combustion reaction occurs, chemical energy is converted to thermal energy, and the temperature of the system rises
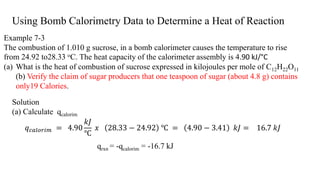
- 5. Using Bomb Calorimetry Data to Determine a Heat of Reaction Example 7-3 The combustion of 1.010 g sucrose, in a bomb calorimeter causes the temperature to rise from 24.92 to28.33 oC. The heat capacity of the calorimeter assembly is 4.90 kJ/°C (a) What is the heat of combustion of sucrose expressed in kilojoules per mole of C12H22O11 (b) Verify the claim of sugar producers that one teaspoon of sugar (about 4.8 g) contains only19 Calories. Solution (a) Calculate qcalorim 𝑞 𝑐𝑎𝑙𝑜𝑟𝑖𝑚 = 4.90 𝑘𝐽 ℃ 𝑥 28.33 − 24.92 ℃ = 4.90 − 3.41 𝑘𝐽 = 16.7 𝑘𝐽 qrxn = -qcalorim = -16.7 kJ
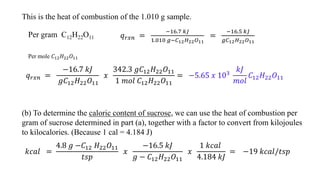
- 6. This is the heat of combustion of the 1.010 g sample. Per gram C12H22O11 𝑞 𝑟𝑥𝑛 = −16.7 𝑘𝐽 1.010 𝑔−𝐶12 𝐻22 𝑂11 = −16.5 𝑘𝐽 𝑔𝐶12 𝐻22 𝑂11 Per mole 𝐶12 𝐻22 𝑂11 𝑞 𝑟𝑥𝑛 = −16.7 𝑘𝐽 𝑔𝐶12 𝐻22 𝑂11 𝑥 342.3 𝑔𝐶12 𝐻22 𝑂11 1 𝑚𝑜𝑙 𝐶12 𝐻22 𝑂11 = −5.65 𝑥 103 𝑘𝐽 𝑚𝑜𝑙 𝐶12 𝐻22 𝑂11 (b) To determine the caloric content of sucrose, we can use the heat of combustion per gram of sucrose determined in part (a), together with a factor to convert from kilojoules to kilocalories. (Because 1 cal = 4.184 J) 𝑘𝑐𝑎𝑙 = 4.8 𝑔 −𝐶12 𝐻22 𝑂11 𝑡𝑠𝑝 𝑥 −16.5 𝑘𝐽 𝑔 − 𝐶12 𝐻22 𝑂11 𝑥 1 𝑘𝑐𝑎𝑙 4.184 𝑘𝐽 = −19 𝑘𝑐𝑎𝑙/𝑡𝑠𝑝
- 7. Problem statement A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol. Solution Since this is a combustion reaction, heat flows from the system to the surroundings- thus, it is exothermic. The heat released by the reaction will be absorbed by two things: (a) the water in the calorimeter and (b) the calorimeter itself. The temperature change of the calorimeter is the same as the temperature change for water.
- 8. a. Calculate the heat absorbed by the water (qwater) b. Calculate the heat absorbed by the calorimeter (qcal)
- 9. The TOTAL heat absorbed by the water and the calorimeter = (a) +(b): =41.2 + 6.86 = + 48.1 kJ. (Remember, q is positive because the heat is being absorbed). The amount of heat released by the reaction = the amount of heat absorbed by the water and the calorimeter. qreaction = – 48.1 kJ 1.000 gram of octane was burned, the heat of combustion for octane = to – 48.1 kJ/gram What is the heat of combustion in kJ/mol? = -48.1 kJ/g x 114 g/mol = – 5483 kJ/mol.
- 10. Work Work involved in the expansion or compression of gases is called pressure volume work. This type of work is performed: i) by explosives, and ii) by the gases formed in the combustion of gasoline in an automobile engine. Consider the decomposition of potassium chlorate to potassium chloride and oxygen. 2𝐾𝐶𝑙𝑂3 → 2𝐾𝐶𝑙 + 3𝑂2 The pressure inside the reaction vessel exceeds the atmospheric pressure and the piston is lifted, the system does work on the surroundings. The work can be calculated by work (w) = force (M * g) * distance (∆h) = -M * g * ∆h 𝑤 = − 𝑀 𝑥 𝑔 𝐴 𝑥 ∆ℎ 𝑥 𝐴 = − 𝑃𝑒𝑥𝑡 ∆𝑉 (7.11)
- 11. Bomb Calorimetry Used to measure the heat evolved in a combustion reaction. The system is everything within the double- walled outer jacket of the calorimeter. This includes the bomb and its contents, the water in which the bomb is immersed, the thermometer, the stirrer, and so on. The system is isolated from its surroundings. . 𝒒 𝒓𝒙𝒏 = − 𝒒 𝒄𝒂𝒍𝒐𝒓𝒊𝒎 𝒘𝒉𝒆𝒓𝒆 𝒒 𝒄𝒂𝒍𝒐𝒓𝒊𝒖𝒎 = 𝒒 𝒃𝒐𝒎𝒃 + 𝒒 𝒘𝒂𝒕𝒆𝒓 … qcalorim = heat capacity of calorium * ∆T When the combustion reaction occurs, chemical energy is converted to thermal energy, and the temperature of the system rises Determination of ΔU and ΔH for Chemical Reactions
- 12. In a bomb calorimeter, the reaction is carried out at constant volume. The motivation for doing so is that if dV = 0, ΔU = qV. Therefore, a measurement of the heat flow normalized to 1 mole of the specified reaction provides a direct measurement of ΔUR. no heat flow will occur between the system and surroundings, and q = 0. Because the combustion experiment takes place at constant volume, w = 0. Therefore, ΔU= 0. So, it is an isolated system of finite size
- 13. Consider the system as consisting of three subsystems: 1. the reactants in the calorimeter, 2. the calorimeter vessel, 3. the inner water bath. These three subsystems are separated by rigid diathermal walls and are in thermal equilibrium. Energy is redistributed among the subsystems as reactants are converted to products, the temperature of the inner water bath changes, and the temperature of the calorimeter changes. ΔT, the change in the temperature of the three subsystems. The mass of water in the inner bath, mH2O; its molecular weight, MH2O ; its heat capacity, CP,m (H2O); the mass of the sample, mS ; and its molecular weight, MS, are known. ΔUcombustion is defined per mole of the combustion reaction, but because the reaction includes exactly 1 mole of reactant, the factor mS / MS in Equation (4.21) is appropriate.
- 14. Example 4.3 When 0.972 g of cyclohexane undergoes complete combustion in a bomb calorimeter, of the inner water bath is 2.98°C. For cyclohexane, ΔUcombustion is –3913 kJ mol-1. Given this result, what is the value for ΔUcombustion for the combustion of benzene if ΔT is 2.36°C when 0.857 g of benzene undergoes complete combustion in the same calorimeter? The mass of the water in the inner bath is 1.812 x 103 g and the CP,m of water is 75.3 J K-1 mol-1. Solution To calculate the calorimeter constant through the combustion of cyclohexane, we write Equation (4.21)
- 15. In calculating ΔUcombustion for benzene, we use the value for Ccalorimeter: Once ΔUcombustion has been determined, ΔHcombustion can be determined
- 16. For reactions involving only solids and liquids, ΔU >> Δ(PV) and ΔH ≈ ΔU. If some of the reactants or products are gases, the small change in the temperature that is measured in a calorimetric experiment can generally be ignored and ΔHcombustion = Δ Ucombustion = ΔnRT ΔHcombustion = Δ Ucombustion + ΔnRT Δn is the change in the number of moles of gas in the overall reaction. and Δn = –3. Note that at T = 298.15 K, the most stable form of C6H12 and H2O is liquid. For this reaction, ΔUcombustion and ΔHcombustion differ by only 0.2%.
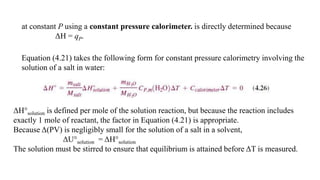
- 17. at constant P using a constant pressure calorimeter. is directly determined because ΔH = qP. Equation (4.21) takes the following form for constant pressure calorimetry involving the solution of a salt in water: ΔH°solution is defined per mole of the solution reaction, but because the reaction includes exactly 1 mole of reactant, the factor in Equation (4.21) is appropriate. Because Δ(PV) is negligibly small for the solution of a salt in a solvent, ΔU°solution = ΔH°solution The solution must be stirred to ensure that equilibrium is attained before ΔT is measured.
- 18. Using Bomb Calorimetry Data to Determine a Heat of Reaction Example The combustion of 1.010 g sucrose, in a bomb calorimeter causes the temperature to rise from 24.92 to28.33 oC. The heat capacity of the calorimeter assembly is 4.90 kJ/°C (a) What is the heat of combustion of sucrose expressed in kilojoules per mole of C12H22O11 (b) Verify the claim of sugar producers that one teaspoon of sugar (about 4.8 g) contains only19 Calories. Solution (a) Calculate qcalorim 𝑞 𝑐𝑎𝑙𝑜𝑟𝑖𝑚 = 4.90 𝑘𝐽 ℃ 𝑥 28.33 − 24.92 ℃ = 4.90 𝑥 3.41 𝑘𝐽 = 16.7 𝑘𝐽 qrxn = -qcalorim = -16.7 kJ
- 19. This is the heat of combustion of the 1.010 g sample. Per gram C12H22O11 𝑞 𝑟𝑥𝑛 = −16.7 𝑘𝐽 1.010 𝑔−𝐶12 𝐻22 𝑂11 = −16.5 𝑘𝐽 𝑔𝐶12 𝐻22 𝑂11 Per mole 𝐶12 𝐻22 𝑂11 𝑞 𝑟𝑥𝑛 = −16.7 𝑘𝐽 𝑔𝐶12 𝐻22 𝑂11 𝑥 342.3 𝑔𝐶12 𝐻22 𝑂11 1 𝑚𝑜𝑙 𝐶12 𝐻22 𝑂11 = −5.65 𝑥 103 𝑘𝐽 𝑚𝑜𝑙 𝐶12 𝐻22 𝑂11 (b) To determine the caloric content of sucrose, we can use the heat of combustion per gram of sucrose determined in part (a), together with a factor to convert from kilojoules to kilocalories. (Because 1 cal = 4.184 J) 𝑘𝑐𝑎𝑙 = 4.8 𝑔 −𝐶12 𝐻22 𝑂11 𝑡𝑠𝑝 𝑥 −16.5 𝑘𝐽 𝑔 − 𝐶12 𝐻22 𝑂11 𝑥 1 𝑘𝑐𝑎𝑙 4.184 𝑘𝐽 = −19 𝑘𝑐𝑎𝑙/𝑡𝑠𝑝
- 20. Problem statement A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol. Solution Since this is a combustion reaction, heat flows from the system to the surroundings- thus, it is exothermic. The heat released by the reaction will be absorbed by two things: (a) the water in the calorimeter and (b) the calorimeter itself. The temperature change of the calorimeter is the same as the temperature change for water.
- 21. a. Calculate the heat absorbed by the water (qwater) b. Calculate the heat absorbed by the calorimeter (qcal)
- 22. The TOTAL heat absorbed by the water and the calorimeter = (a) +(b): =41.2 + 6.86 = + 48.1 kJ. (Remember, q is positive because the heat is being absorbed). The amount of heat released by the reaction = the amount of heat absorbed by the water and the calorimeter. qreaction = – 48.1 kJ 1.000 gram of octane was burned, the heat of combustion for octane = to – 48.1 kJ/gram What is the heat of combustion in kJ/mol? = -48.1 kJ/g x 114 g/mol = – 5483 kJ/mol.
Editor's Notes
- Exothermic reactions, the heat of reaction is a negative quantity (qrxn < 0)
- This quantity of heat, in turn, is just the negative of the thermal energy gained by the calorimeter and its contents
- A combustion reaction is an exothermic reaction, which means that energy flows, in the form of heat, from the reaction system to the surroundings. Therefore, the q for a combustion reaction is negative. 1 Calorie is equivalent to 1 kilocalorie; the capital C in Calories denotes kcal on food labels,
- The temperature change of the calorimeter is the same as the temperature change for water. In this step, however, we must use the heat capacity of the calorimeter, which is already known. When using heat capacity, the mass of the calorimeter is not required for the calculation. (It’s already incorporated into the heat capacity).
- molecular mass of octane = 114.23 g/mol
- Suppose that this decomposition is carried out in a vessel. The walls of the container resist moving under the pressure of the expanding except for the piston that closes off the cylindrical top of the vessel.
- This quantity of heat, in turn, is just the negative of the thermal energy gained by the calorimeter and its contents
- Diathermal wall between two thermodynamic systems allows heat transfer but not mass transfer across it. However, to determine , the heat capacity of the calorimeter, Ccalorimeter, must first be determined by carrying out a reaction for which is ΔUR already known,
- A combustion reaction is an exothermic reaction, which means that energy flows, in the form of heat, from the reaction system to the surroundings. Therefore, the q for a combustion reaction is negative. 1 Calorie is equivalent to 1 kilocalorie; the capital C in Calories denotes kcal on food labels,
- The temperature change of the calorimeter is the same as the temperature change for water. In this step, however, we must use the heat capacity of the calorimeter, which is already known. When using heat capacity, the mass of the calorimeter is not required for the calculation. (It’s already incorporated into the heat capacity).
- molecular mass of octane = 114.23 g/mol