Change control
- 2. Table of content 1. Definitions ( Change and Change Control) 3 2. Tasks of Change Control 4 3. Principles of Change Control 5 4. Regulatory requirements 6 5. Elements of Change Control 7 6. Steps of Change Control 8 7. Classification of Change Control with example 9-10 8. Motives for Change Control 11 9. Documentation 12-13 10. Challenges of Change Control 14-16 11. Examples of Changes 17-18 07/15/13 2
- 3. CHANGE CONTROL • Change refers to any modification in equipment, manufacturing materials, facilities, utilities, design, formulations, processes, packaging/labeling, computer systems, and all associated documentation (SOPs, quality manual, etc.). • According to WHO “Change control is a formal system by which qualified representatives of appropriate disciplines review proposed or actual changes that might effect a validated status. The intent is to determine the need for the action that would ensure that the system is maintained in a validated state”. OR • Change Control within a QMS is a formal process used to ensure that a change to a system is introduced in a controlled and coordinated manner. 07/15/13 3
- 4. TASKS OF CHANGE CONTROL • Change control minimizes the risk that changes can have on the quality or process characteristics • Each change to previously approved requirements requires a review and authorization to keep the system in its original state of “proven suitability” • Formal change control guarantees that all changes are evaluated for their effect on product quality or validation status • Change control programs have become recognized as essential element of the pharmaceutical quality assurance system 07/15/13 4
- 5. PRINCIPLES OF CHANGE CONTROL • As the change control is considered an essential element of the pharmaceutical quality assurance system, it is logical the person responsible is quality assurance (QA representative, QA head) • Change control is not department-specific, rather the task of the whole company • The change control monitors all types of changes which can influence the process or product quality and states the measures necessary for implementing the change or decides that a change should not be implemented 07/15/13 5
- 6. REGULATORY REQUIREMENTS • Any changes in the production and processes must be controlled-meaning recorded, reviewed and approved by the QC unit. • Manufacturers certified to ISO 9000:2000 and ISO 13485 standards are required to ensure that any changes affecting QMS ( including product requirements, deign and development changes) should be controlled. • All changes should be made according to approved (approval implies successful testing or thorough review/investigation) written company policies and procedures. • Change control procedures have to be written as a way of standardizing instructions. • In FDA and ISO environments, strict adherence to approved policies and procedures is a key factor in keeping manufacturing operations in a state of control and it is what makes change control crucial. 07/15/13 6
- 7. ELEMENTS OF CHANGE CONTROL • INITIATOR Change is typically introduced by a initiator or originator. Initiating a changes involves filling out a change request form which them moves through a process or system of review and approval. • CHANGE CONTROL COMMITTEE May be a single entity for the whole company or may be one for the company’s manufacturing site. It usually includes representatives from different departments involved in production such as quality, manufacturing, regulatory affairs and engineering. Depending on the change, committee may include legal, sales and marketing departments. • CHANGE ADMINISTRATOR In pharmaceutical companies, cGMP requires that all changes must be reviewed and approved by quality unit. In such companies, there must be a “Change Administrator”, a role usually assumed by quality unit. 07/15/13 7
- 8. STEPS OF CHANGE CONTROL • Record / Classify ( Initiator initiates a change by filling a change request form. The change control team then records and categorize it). • Assess (Assessors then make their risk analysis typically by answering a set of questions concerning risk, both to the business and to the process, and follow this by making a judgment on who should carry out the change, then it is sent for planning ). • Plan ( Planning team plans the change in detail as well as construct a regression plan in case if the change is backed out). • Build / Test ( team then make solutions, which will then be tested. They will then seek approval and request a time and date to carry out the implementation phase) . • Implement ( In this phase, finalized solutions or changes are implemented). • Evaluation ( after change has been implemented, it should be evaluated) • Close / Gain Acceptance ( when change is implemented correctly then it will be closed). 07/15/13 8
- 9. CLASSIFICATION OF CHANGES • A classification procedure nay help in determining the level of testing, validation and documentation needed to justify the changes of validated process. • Changes may be classified as Major or Minor, depending on the nature and extent of changes or the effect these changes impart on process. • They can also be categorized as specification changes, raw material changes, equipment changes. 07/15/13 9
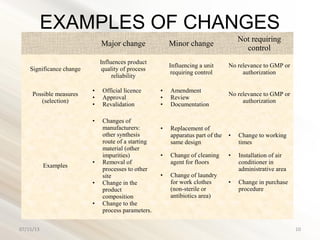
- 10. EXAMPLES OF CHANGES Major change Minor change Not requiring control Significance change Influences product quality of process reliability Influencing a unit requiring control No relevance to GMP or authorization Possible measures (selection) • Official licence • Approval • Revalidation • Amendment • Review • Documentation No relevance to GMP or authorization Examples • Changes of manufacturers: other synthesis route of a starting material (other impurities) • Removal of processes to other site • Change in the product composition • Change to the process parameters. • Replacement of apparatus part of the same design • Change of cleaning agent for floors • Change of laundry for work clothes (non-sterile or antibiotics area) • Change to working times • Installation of air conditioner in administrative area • Change in purchase procedure 07/15/13 10
- 11. MOTIVES FOR CHANGE CONTROL • Experiences from developmental and transfer phases. • Results of process monitoring and statistical process control. • Finding from quality control and stability results. • Continuous improvement processes and processes optimization. • Strategic consideration ( changing suppliers, product transfer) • CAPA processes • Customer requests • Regulations ( legal changes and official requirements) 07/15/13 11
- 13. DOCUMENTATION • Change control requires a written procedure to establish at least the following steps: – What type of changes does change control take into account? – What are the requirements for urgent changes? – For which areas does this operating procedure apply? – Who can suggest changes? – How are changes requested (forms, method of communication)? – How are changes graded and who is responsible for the rating? – How are the measures for carrying out the change established? – Who is responsible for the implementation and monitoring of all measures? – Who is included in the change control team? – What are the duties of the change control team? – How is the change documented (format, content, storage)? – Who is responsible to authorize changes? • All quality-relevant changes should be documented • All actions to be taken, including the need for and extent of qualification or validation, should be described • The records can be archived in paper form or electronically • When storing documents, raw data and other relevant documents for change should be kept accessible • The documentation for the change procedure should show that the change was evaluated (risk analysis) 07/15/13 13
- 14. What makes change control so challenging? 07/15/13 14
- 15. CHALLENGES IN CHANGE CONTROL There are many factors, but the following are the most common issues: • Poor Communication - Lack of communication between departments, failure to follow up or escalate change requests and delayed or inappropriate notification of changes made by suppliers etc. • Poor Turnaround - Timeliness can make or break a product's chances of succeeding in the market. Companies that rely on a manual system to generate data and to route and track submissions and change orders are likely to have poor turnaround for change implementation because the system requires more time and effort. The problem becomes worse when there is an unexpected change—a deviation—and supporting data is not generated in a timely manner. Delays may also result because of failure to recognize the urgency of a change and the absence of a contingency plan for certain types of changes. 07/15/13 15
- 16. • Ineffective Documentation - A manual system makes it more difficult to update documentation, including revision history, and to find/retrieve necessary data to support a change. The proposed change may be delayed when an outdated record does not show revision history, or testing may be duplicated because documentation has not been updated with results of a previous testing. • Training not integrated with Change - Adequate training of personnel is a prerequisite in both FDA and ISO environments. When training control is not connected to the rest of the quality process, such as in manual systems, keeping training current with procedures that have changed can be difficult. Training tasks can easily fall through the cracks if employees are not vigilant in seeking them. 07/15/13 16
- 17. EXAMPLES OF CHANGES 07/15/13 17
- 18. Examples of changes Changes to the cleaning procedure - use of a new cleaning agent - change in concentration / volumes of cleaning agent - change in volumes of rinsing water - change of cleaning process parameters Changes to the production equipment - changes to the CIP equipment - changes to / replacement of equipment parts (difficult to give detailed examples as these changes are too diverse) - change of process parameters Changes to the product - changes to composition (reformulation with different excipients) - changes to the manufacturing process (i.e. transfer of a product) Changes to HVAC system / unidirectional flow Changes in lay-out (i.e. implementation of a pass-box07/15/13 18