Chapter 1 : Rate of Reaction
- 2. Rate of reaction Rate of reaction is the measurement of the speed which reactants are converted into products in a chemical reaction / change in a selected quantity during a reaction per unit time. Average rate of reaction is the average value of the rate of reaction over an interval of time. Instantaneous rate of reaction / Rate of reaction at a given time are the actual rate of reaction at that instant.
- 4. Total surface area of solid reactant Concentration of reactant Temperature of reactant Use of catalyst Pressure of gaseous reactant
- 7. Take note :
- 8. The smaller the size (increase the total surface area), cm3, of the solid reactant, the higher the rate of reaction, cm3 s-1 or cm3 min-1. Explanation:
- 9. Effect of concentration of a liquid reactant on the rate of reaction The higher the concentration, mol dm-3, of a liquid reactant, the higher the rate of reaction, mol dm-3 s-1 or mol dm-3 min-1.
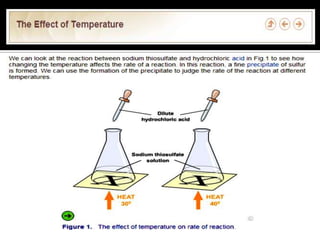
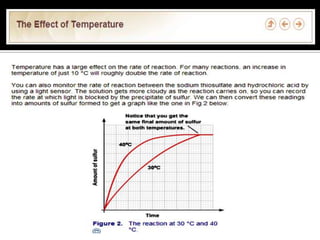
- 12. Increase in temperature, the higher the rate of reaction.
- 13. Alters the rate of reaction It is specific in its action. It can only catalyse a particular reaction Does not change the quantity of products formed Only small amount of catalyst is needed to increases the rate of reaction. (An increase in the quantity of catalyst will increase the rate of reaction but only a very slight increase.) Catalyst remains chemically unchanged but may undergo physical changes.
- 16. (catalyst)
- 18. catalyst
- 19. Effect of pressure on the rate of reaction • Increase in pressure, the higher the rate of reaction (reversible reaction and gaseous reactants and gaseous product).
- 21. 1.3 Collision Theory • According to kinetic theory of matter : Particles of matter are in continuous motion & constantly in collision with each other. • Collision theory states a reaction occur when the particle of the reactant collide with each other with the correct orientation and achieve activation energy. • Collision theory also states that the rate of reaction can be determined by frequency of collision / number of collisions per second & magnitude of Ea. • Collision which achieve a minimum amount of activation energy & with correct orientation will result in reaction. (Effective collision) • Ineffective collision is the particles that collide with energy less than activation energy or wrong orientation.
- 22. Activation energy, Ea • The energy barrier that must be overcome by the colliding particles of the reactants so that the reaction can occur. • Can be visualized by sketching the energy profile diagrams.