chemical bonding and molecular structure
- 1. Ch em Pr is tr oj y ec W t or k A Akarshik Banerjee Pratyush Dey Sayantan Biswas Class- X l ’B’
- 2. P Matter is made up of on or different elements. But under normal conditions except noble gasses no other elements occur as single atom. Evidently there is a force which holds together various constituent particles in different chemical species. This force is called the-
- 3. S Octet Rule As per electronic theory of chemical bonding atoms combine to attain noble gas configuration. Covalent Bond When two or more atoms share electron pairs they are said to be covalently bonded.
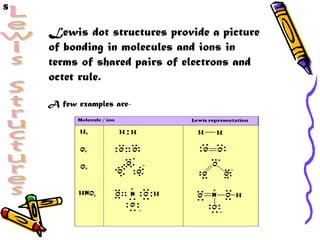
- 4. S Lewis dot structures provide a picture of bonding in molecules and ions in terms of shared pairs of electrons and octet rule. A few examples are-
- 5. P Formal charge on each O atom are:On O no.1= 6 - ½ - ½ (6)=1 On O no. 2= 6 – 4 – ½ (2)=0 On O no.3= 6 – 6 – ½ (2)= -1
- 6. A The incomplete octet of central atom. Odd electron molecules. The expanded Octet. When two or more elements form a bond by complete transfer of electrons the bond is said to be electrovalent bond.
- 7. A ClIt is defined as the energy required to completely separate one mole of solid ionic compound to gaseous constituent Na+ ions. Covalent radius is measured as the radius of an atom’s core which is in contact with the core of adjacent atom in bonded situation. Van der Waals radius represents the overall size of the atom which includes its valence shell in non –bonded situation. Bond angle is the angle between the orbitals containing the electron pairs around the central atom/molecule/ complex ion. Bond enthalpy is the energy required to break one mole of like bonds in two atoms in gaseous state. Bond order is the no. of bonds between two elements in a molecule. rC rv dw
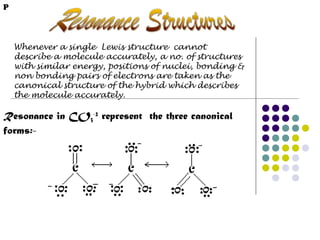
- 8. P Whenever a single Lewis structure cannot describe a molecule accurately, a no. of structures with similar energy, positions of nuclei, bonding & non bonding pairs of electrons are taken as the canonical structure of the hybrid which describes the molecule accurately. Resonance in CO3-2 represent the three canonical forms:-
- 9. S In heterogeneous covalent compounds due to greater electronegativity of one atom the shared electron pair ins displaced more towards it than the other molecule. As a result of polarization the molecule posses a net Dipole moment (depicted as a small arrow with the tail in the positive center and head on the negative center.) Dipole moment = charge x distance of separation By knowing the dipole moment the symmetry or the polarity of a molecule can be known. The net Dipole moment of NF3 is less than NH3 despite fluorine being more electronegative is due to the fact that the arrows are in opposite directions in both cases and the lone pair adds to the dipole moment in the latter but decreases the
- 10. P The shape of a molecule depends upon the no. of valence electrons. Pairs of electrons repel each other. They tend to occupy such positions so as to minimise repulsion and maximise distance between them. The valance shell is taken as a sphere . A multiple bond is treated as a single electron pair and single electron pairs in multiple bonds are treated as a single super pair. The VESPER model is applicable to any such structure where two or more resonance structures can represent a molecule. VSEPR Theory is able to predict the geometry of large no. of molecules especially the compounds of p-block elements accurately.
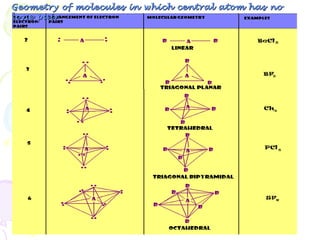
- 11. Geometry of molecules in which central atom has no lone pair
- 12. Geometry of molecules in which central atom has 1 Or more lone pairs
- 13. S As two atoms approach each other the following forces come into action:Attractive forces betweena) Nucleus of an atom & its own electrons. b) Nucleus of one atom & electrons of the other atom. Repulsive forces betweena) Electrons of two atoms. b) Nuclei of two atoms. The valance bond theory explains the directional properties of bond as a consequence of inter electronic repulsion.
- 14. A p-p overlapping (sigma Bond) s-p overlapping (sigma Bond) p-p overlapping (pi Bond) s-s overlapping (sigma Bond) Strengths – The strengths of bonds depends upon the extent of overlap. So sigma bond has more strength than pi bond.
- 15. A Hybridisation is the process of intermixing of the orbitals of slightly different energies in order to redistribute their energies to form new set of orbitals with equivalent energy and shape. S-p hybridisation It involves mixing of 1S orbital and 1p orbital S-p 2 hybridisation It involves mixing of 1S and 2p orbitals.
- 16. A S-p 3 hybridisation It involves mixing of 1S and 3p orbitals. Hybridisation involving d orbitals S-p 3 d hybridisation S-p 3 d 2 hybridisation
- 17. P The electrons in various molecules are present in molecular orbitals. The atomic orbitals of comparable energy and symmetry combine to form molecular orbitals. The molecular orbitals are polycentric. The no. of molecular orbitals formed is equal to the no. of atomic orbitals taking part in combination. The energy of the bonding molecular orbitals are less than the energy of the non bonding orbitals thus the bonding orbitals are more stable. The electron probability distribution around a group of nuclei in a molecule is called a molecular orbital. It obeys aufbau principle , Hund’s rule and Puali’s exclusion principle.
- 18. A In O2 molecule In H2 molecule He2 molecule H2 O2 He 2
- 19. S The hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom of another molecule. When bonded with a strong electronegative atom the hydrogen atom acquires partial positive charge as the electron gets displaced more towards the electronegative atom. This causes formation of polar molecule having electrostatic force of attraction. They can be either:(1)Intermolecular Or, (2)Intramolecular
- 20. The name of the slide designer is on top left corner of every slide: A for Akarshik (rollno. 5) S for Sayantan (rollno. 24) P for Pratyush (rollno. 21) Hope the project was decent……. Thanks for watching.