cycloalkanes
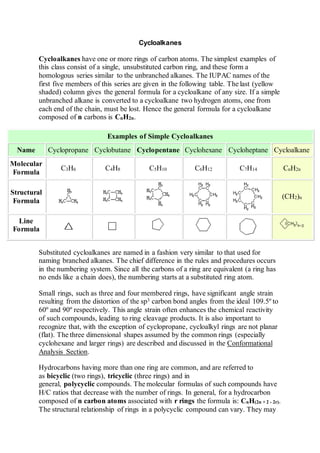
- 1. Cycloalkanes Cycloalkanes have one or more rings of carbon atoms. The simplest examples of this class consist of a single, unsubstituted carbon ring, and these form a homologous series similar to the unbranched alkanes. The IUPAC names of the first five members of this series are given in the following table. The last (yellow shaded) column gives the general formula for a cycloalkane of any size. If a simple unbranched alkane is converted to a cycloalkane two hydrogen atoms, one from each end of the chain, must be lost. Hence the general formula for a cycloalkane composed of n carbons is CnH2n. Examples of Simple Cycloalkanes Name Cyclopropane Cyclobutane Cyclopentane Cyclohexane Cycloheptane Cycloalkane Molecular Formula C3H6 C4H8 C5H10 C6H12 C7H14 CnH2n Structural Formula (CH2)n Line Formula Substituted cycloalkanes are named in a fashion very similar to that used for naming branched alkanes. The chief difference in the rules and procedures occurs in the numbering system. Since all the carbons of a ring are equivalent (a ring has no ends like a chain does), the numbering starts at a substituted ring atom. Small rings, such as three and four membered rings, have significant angle strain resulting from the distortion of the sp3 carbon bond angles from the ideal 109.5º to 60º and 90º respectively. This angle strain often enhances the chemical reactivity of such compounds, leading to ring cleavage products. It is also important to recognize that, with the exception of cyclopropane, cycloalkyl rings are not planar (flat). The three dimensional shapes assumed by the common rings (especially cyclohexane and larger rings) are described and discussed in the Conformational Analysis Section. Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with r rings the formula is: CnH(2n + 2 - 2r). The structural relationship of rings in a polycyclic compound can vary. They may
- 2. be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table. Examples of Isomeric C8H14 Bicycloalkanes Isolated Rings Spiro Rings Fused Rings Bridged Rings No common atoms One common atom One common bond Two common atoms Ring Strain Cycloalkanes tend to give off a very high and non-favorable energy, and the spatial orientation of the atoms is called the ring strain. When atoms are close together, their proximity is highly unfavorable and causes steric hindrance. The reason we do not want ring strain and steric hindrance is because heat will be released due to an increase in energy; therefore, a lot of that energy is stored in the bonds and molecules, causing the ring to be unstable and reactive. Another reason we try to avoid ring strain is because it will affect the structures and the conformational function of the smaller cycloalkanes. One way to determine the presence of ring strain is by its heat of combustion. By comparing the heat of combustion with the value measured for the straight chain molecule, we can determine the stability of the ring.
- 3. The figures below show cyclopropane, cyclobutane, and cyclopentane, respectively. Cyclopropane is one of the cycloalkanes that has an incredibly high and unfavorable energy, followed by cyclobutane as the next strained cycloalkane. Any ring that is small (with three to four carbons) has a significant amount of ring strain; cyclopropane and cyclobutane are in the category of small rings.A ring with five to seven carbons is considered to have minimal to zero strain, and typical examples are cyclopentane, cyclohexane, and cycloheptane. However, a ring with eight to twelve carbons is considered to have a moderate strain, and if a ring has beyond twelve carbons, it has minimal strain. The Baeyer Theory on the Strain in Cycloalkane Rings Many of the properties of cyclopropane and its derivatives are similar to the properties of alkenes. In 1890, the famous German organic chemist, A. Baeyer, suggested that cyclopropane and cyclobutane derivatives are different from cyclopentane and cyclohexane, because their C—C—C angles cannot have the tetrahedral value of 109.5°. At the same time, Baeyer hypothesized that the difficulties encountered in synthesizing cycloalkane rings from C7 upward was the result of the angle strain that would be expected if the large rings were regular planar polygons (see Table 12-3). Baeyer also believed that cyclohexane had a planar structure like that shown in Figure 12-2, which would mean that the bond angles would have to deviate 10.5° from the tetrahedral value. However, in 1895, the then unknown chemist H. Sachse suggested that cyclohexane exists in the strain-free chair and boat forms
- 4. Compound n Angle Strain at each CH2 Heat of Combustion ΔHo(kcal/mo l) Heat of Combustio n ΔHo per CH2/N(kc al/mol) Total Strain (kcal/mol) ethene 2 109.5 337.2 168.6 22.4 cyclopropane 3 49.5 499.9 166.6 27.7 cyclobutane 4 19.5 655.9 164.0 26.3 cyclopentane 5 1.5 793.4 158.7 6.5 cyclohexane 6 10.5 944.8 157.5 0.4 cycloheptane 7 19.1 1108.1 158.4 6.3 cyclooctane 8 25.5 1268.9 158.6 9.7 cyclononane 9 30.5 1429.5 158.8 12.9 cyclodecane 10 34.5 1586.1 158.6 12.1 cyclopentadecane 15 46.5 2362.5 157.5 1.5 open chain alkane 157.4 - Types of strain There are different types of ring strain, such as those mentioned earlier. Trans annular strain is defined as the crowding of the two groups in a ring. There is also eclipsing strain, also known as torsional strain, which means that the intramolecular strain present is due to the bonding interaction between two eclipsed atoms or groups. Another type of strain is called the bond angle strain, and it is present when there is a poor overlap between the atoms. There must be an ideal bond angle to achieve the maximum bond strength and that will allow the overlapping of the atomic orbitals.