Empowering Indian pharmaceutical industry to lead globally.
- 1. White Paper on Empowering Indian Pharmaceutical Industry to lead Globally Dr. R. B. Smarta Managing Director Interlink Marketing Consultancy Pvt. Ltd Insights For Business Results
- 2. In life you have either reasons or results… In life you have either reasons or results…
- 3. 1. Preface 2. Focus on 'Globally Leadership' 3. Insights and opportunities in global Pharma industry 4. Agenda for Policy makers , Industry and Stakeholders 5. References 3 4 11 16 19 Content 1 Page
- 5. Preface Interlink's knowledge team was privileged to undertake preparation of white paper on “empowering Indian pharmaceutical industry to lead globally”. Vast experience of our consultants of Pharma industry, embedded with consultancy perspective, contributions of our research team and insights of our internal consultants have made this white paper insightful andmeaningful. Our findings emerge from an in-depth review of numerous reports, intense discussion at various brainstorming sessions and sharpening of our thought process relating to the central themeofthiswhitepaper. We had the benefit of receiving well considered suggestions and comments during the preparation of this white paper from Mr. Rajendra Gupta from policy perspective, Dr. Navneet Wadhwa from medical perspective, Mr. S. W. Deshpande from regulatory perspective, Mr. Sanjiv Bosamia from innovation perspective, Ms. Anju Ghangurde and Ms. Viveka Roychowdhury from industry and editorial perspective, Mr. Subodh Priolkar from pharmacy perspective and Ms.Ruth D'Souza from consulting perspective to further sharpen this White Paper. We also extend our thanks to Mr. Satya Brahma - Chairman - 3rd Annual Pharmaceutical LeadershipSummit2010afterdiscussionsinthecontextofthisWhitePaper. We acknowledge the efforts of Interlink Consulting Team, Ms. Kirti Wadekar, Mr. Sachin AdawadeandMr.SarangPathakwhilepreparingtheWhitepaper. We trust that the White Paper will provide clues for actions for policy makers as well as industry andotherstakeholderstoleadIndiaglobally. Dr. R. B. Smarta Managing Director Interlink Marketing Consultancy Pvt. Ltd 1.Preface 3
- 6. Over the past decade pharmaceutical companies have had to navigate a rapidly changing and challenging environment, in which all stakeholders have experienced significant pressures to change. This change is taking place world wide, leaving India with a level playing field. Product pipelines are thin everywhere. Markets are changing - key dynamics and demographics are changing - and India has acquired an unique space with more opportunities than ever before to become a global leader. The industry's current business models are overused and economically unsustainable. They are operationally unsuited to the kind of quick response that is necessary for generating revenue in different markets. The need of the hour is a bold new vision. This vision can originate from a country specific strategy for the Pharma industry and India as a country needs to approach from that angle. Only leaders who have the willingness to embrace a fundamentally new approach to their business will lead the world and India has a chance to lead. Over time theAmerican Healthcare system has been driven more by costs and not as much by the outcome which is evident from the 17% of GDP spend on healthcare. In spite of being 31st in life expectancy and 40th in child mortality among all nations, 77% ofAmericans have at least one chronic disease. The US healthcare system which ranks 37th amongst nations in terms of effectiveness is undergoing reforms. The world economic downturn has pressurized all nations to bring down their healthcare costs and reform healthcare systems. Like the US, most nations are in a mood to introduce healthcare reform since the key parts of healthcare bills are COSTS - corporate healthcare costs and health insurance costs. The effort by governments worldwide to curtail mounting health care costs presents another spur to growth. Spending on pharmaceutical products remains a minor but highly visible component of total healthcare costs across geographies. Therefore, the search for less expensive therapeutic alternatives is gaining importance, and generic drugs are an appropriate option. Countries across the world are now initiating reforms to encourage the use of generic drugs to curb increasing individual health care outlays. In recent times, there has been a rapid reemergence of the generic drug industry in developed countries such as the US, Western Europe, and Japan, largely because of the World review 'Generics' - the new savior of the world 2. Focuson'GloballyLeadership' 4
- 9. Asia is the largest region importing pharmaceuticals accounting for 30% of India's pharmaceutical exports followed by Europe (24%) and North America (21%). During 2008- 09 the United States of America was the top export destination with a share of approx. 18% in India's pharmaceutical exports valued at USD 1.55bn, followed by Russia valued at USD 0.33bn with a share of 3.84%, Germany (USD.31bn. and 3.65%),Austria (USD 0.31bn. and 3.58%) and UK (USD 0.27bn. and 3.12%). The country has 814 WHO CGMP approved pharmaceutical plants (as on Apr. 2009 according to the DCGI website). It has 153 European Directorate of Quality Medicine (EDQM) approved pharmaceutical manufacturing facilities. ll Indian companies have an opportunity to lead the world provided they are supported to seize it. A national direction for attaining world leadership in generics needs to be set involving all aspects like policy, regulatory, market dynamics and marketing mix. In the last World Congress of Alternative Medicine in Mumbai, conducted in January 2007, revealing statistics were presented: 97% of the world population uses alternative and traditional medicine and only 7% uses allopathic medicine. This is an area in which India has many healthcare solutions to offer to the world. The project of establishing “National Formularies” by the Ayurveda, Siddha and Unani Pharmacopoeial Committee could serve as the first phase to unify and standardize alternate medicines. The establishment of Pharmacopoeial standards will benefit humanity and reposition the Indian Pharmaceutical Industry as a provider of total Healthcare solutions. Today a large segment of the population - the educated in particular - is willing to choose Alternate Medicines / Supplements Risk Factor % of deaths attributed to risk % of disease burden attributed to risk factor Low weight 13% 15% Blood pressure 7% 2% Cholesterol 5% 2% Low fruit and vegetable intake 4% 1% Zinc deficiency 3% 3% Vitamin A deficiency 3% 3% Iron deficiency 3% 3% Obesity 2% 1% 7
- 10. from a wide variety of alternative therapies, such as Nutraceuticals, Aromatherapy, Ayurveda, and Homeopathy. There is growing awareness of the 15 leading risk factors for high mortality rates, eight of which are nutrition linked factors like low weight, blood pressure, cholesterol, low fruit and vegetable intake, zinc deficiency, vitamin A deficiency, iron deficiency, and obesity. People are willing to go in for supplements to combat illnesses resulting from these factors. This market is to the tune of USD 117 billion and U.S. Europe, and Japan are the key markets, contributing 60% of the global market. India can aim at capturing a significant share of this newly formed market as India has innate strengths of 16 biodiverse zones, talent to manufacture low cost / high quality ANIs (Active Nutraceutical Ingredients) along with a rich heritage ofAyurvedic drugs. While India is an attractive market segment for global Pharma companies due to its demographic dividend and sheer volumes of market potential, the returns in India may not be commensurate with the investment in a given time, thus not measuring up to expectations. The Indian Healthcare Industry is estimated at USD 22 billion (Rs.1056 Billion). Estimated growth at 13% annually assures a return of 15-20% for healthcare providers. This sector at present employs 4 million people directly or indirectly. The private Healthcare Sector is currently estimated at USD 14.8 billion (Rs. 690 billion). 85% of private sector establishments have less than 25 beds. Specialty and Super specialty hospitals account for 2% of total institutes. Corporate hospitals constitute less than 1% of all institutes. Private sector accounts for 82% of all out patient units and 52% of all hospitalization (CCI Report 2008). Healthcare Infrastructure Private Sector with less than 25 beds Speciality & Super Speciality Corporate Hospitals Rest Private Healthcare Infrastructure in India 12% 1% 2% 85% 8
- 11. Number Of Hospitals 15,393 Public Hospitals 4,049 Private Hospitals 11344 Hospital Beds 875000 Number Of Doctors 592215 Number Of Nurses 737000 Number Of Dentists 80,000 Number of Medical Colleges 170 New Doctors Every year 18000 Number of Pharmacies 4,40,000 In case of Health Infrastructure and Health professionals, India has the following spread: Policy makers in India have to introspect whether the available infrastructure will help India lead and fulfill the expectations of all who are looking at India as a potential market? The lack of patient education, patient compliance, and focus on preventive healthcare, compounded with the need for more healthcare professionals has led to a gap between disease detection and treatment. Increased reach of health insurance and ensuring trained healthcare manpower especially in rural areas is essential. The large pool of pharmacists can be well utilized for improvement of the healthcare delivery mechanism and insurance can be stepped up to ensure greater affordability of the general public for treatment. In order to capitalize on the three major opportunity areas like Generics, Alternate medicines and Health infrastructure, it is essential to empower the Industry through a concerted effort from all stakeholders. For the Indian pharma industry to attain global leadership a shift in the mindset of stakeholders is called for. Pharma industry is becoming the core of healthcare at the delivery level and other parts of Healthcare like diagnostics, infrastructure, radiology are going to grow at a faster page. 1. While the Pharmaceutical industry may remain a mere 10% of total healthcare costs, it will not operate in isolation. It will play a central role provided all Stakeholders understand its centrality and carefully create value through new inclusive business models. In sum 9
- 12. 2. A combined approach towards Health comprising of medicine + lifestyle + wellness will create a role for alternative medicine. Besides allopathy, other branches like Nutraceuticals, Homeopathy, Unani, Ayurveda, Yoga, Life-Style and Biosimilars may be recognized for promoting wellness to initiate an approach to holistic medicines and develop new regulation. 3. The paradigm of managing health should be widened from diagnostics, treatment and healthy habit formation to active prevention of disease. 4. Innovation of new medical entities need not be limited and could come from biology, technology and genomics. 5. All patents must be issued on significantly different established outcomes and not on mere marginal improvements. 10
- 13. Key market dynamics Near term growth prospects in the U.S. have strengthened reflecting both sustained levels of price increases and changing inventory stocking patterns. Pharmacy chains are managing their inventory levels tightly based on expectations of patient demand, which has led to a greater purchasing volatility. While payors seek to limit price increases and boost the use of lower-cost generics, pharmaceutical manufacturers are expected to maintain their pricing practices, competing on the basis of clinical evidence and value. Growth has slowed in countries where there was high out of pocket spending on pharmaceuticals and steep declines in macroeconomic activity, especially in Russia, Mexico and South Korea.At the same time, growth has been less affected to date in countries where drugs are largely funded publicly such as in Germany, Japan and Spain. Consistent with trends of the past several years, the next five are expected to reflect a significant imbalance between new product introductions and patent losses. This is the primary factor limiting global pharmaceutical market growth to the mid single digits through 2013. During the next five years, products that currently generate an unprecedented USD 137 billion in sales are expected to face generic competition. At the same time, new products that will enable innovative approaches for treating patients suffering from diseases such as osteoporosis, respiratory ailments, thrombosis, multiple sclerosis and cancer are not expected to generate the same magnitude of sales as products losing patent protection. Despite economic conditions significantly affecting some markets – notably Russia, Turkey, South Korea and Mexico – the seven pharmerging countries are expected in aggregate to grow by 12 - 14 percent in 2010 and 13 - 16 percent over the next five years. China's pharmaceutical market is expected to continue to grow at a 20+ percent pace annually, and contribute 21 percent of overall global growth through 2013. Russia and Turkey may be impacted significantly by new measures intended to reduce the level of healthcare spending in those two markets. Growth prospects in the U.S. market improve Economic downturn affects markets to varying degrees Impact of the innovation/patent loss imbalance dampens growth prospects Pharmerging markets in aggregate sustain strong growth 3. InsightsandopportunitiesinglobalPharmaindustry 11
- 14. Healthcare access and funding under intensifying pressure Impact of healthcare reforms in the world Role of generics and patented medicines Change in health paradigms: The economic climate has heightened concerns by payors about healthcare funding and intensified their efforts to limit access to non generic drugs. During the next five years, markets will be impacted by numerous payor actions, including the imposition of price cuts on existing drugs, the raising of standards required to achieve reimbursement of innovative therapies and the use of economic incentives for prescribers and pharmacists to drive a shift to generic alternatives. Evidence of the value that medicines bring to healthcare systems will be required to achieve access and funding in both developed and emerging markets. A number of events may occur in 2010 that also could have a long-term effect on the pharmaceutical market. These include the potential for passage of comprehensive healthcare reform as well as legislative or regulatory actions in different countries. Much depends on the magnitude, timing and extent of the global economic recovery. Role of generics will make immense sense in healthcare delivery all over the world. Patented medicines, customized medicines, tele-medicines and high order disease dependent medicines will capture a special niche from this market. All over the world the fulcrum will shift from sickness to prevention of disease. Markets will be thrown wide open with opportunities pertaining to both sick and healthy consumers. India's response to market dynamics The prices of drugs in Indian markets are lower as compared to world prices borne out by the fact that in spite of ranking 3rd globally in volume sales, India ranks a mere 14th in size of the industry. This is fundamentally due to the absence of a separate payor system. The cost of API's is low and provides an edge for affordable pricing. The orientation of Indian Manufacturers to invest in patents is low compounded with the pressure on profits. The larger also public expects cost effective medication. Indian policy makers thus want to make medicines affordable and available. The absence of a patent regime in the past gave an opportunity to India to get into a greater generic mindset and it delivered a low cost healthcare system. 12
- 15. With publics and governments across the world pushing for a more transparent pricing process in an endeavor to bring down public spending on healthcare, the worldwide opportunity for generics will expand in the next 5 to 7 years. Populations all over the world are aging and the nature of the diseases that need treatment to care for these people is changing. Besides lifestyle diseases, growing economies like China, India, and Brazil have different disease patterns. Industry will have to find out cost effective solutions for the world. India can find out such solutions due to its quality of generic and cost efficient mindset. The Indian Pharma Industry was dominated by multinationals in the 1960's and 1970's. 7 out of top 10 organizations were multinationals who controlled the market on the basis of their blockbuster brands. The scenario was reversed in the 1990's, with 7 out of top 10 companies being Indian national companies. The change was aided by empowering policies and regulatory procedures which provided impetus to Indian Manufacturers. This was coupled with the technical brilliance of Indian scientists and technocrats, who used the opportunity provided by the process patents regime to bring imitations of patented products to the Indian market at affordable prices. Moreover the cost of entry and exit in the pharma industry was minimal compared to the global context. The drug policy (revised every 8th year beginning 1971) was designed to increase the availability and affordability of medicines but created pressures on pricing and profitability, forcing the Industry to reinvent itself. In 2005 with the introduction of product patents, the entire domestic market became a generics (or branded generics) market overnight. According to Union Minister of State for Chemicals and Fertilizers, Mr. Srikant Kumar Jena, India tops the world in exporting generic medicines worth of USD 11 billion and currently the Indian pharmaceutical industry is one of the worlds largest and most developed. On the other hand, India is expected to rank among the top 10 global pharmaceutical markets by 2015. The increasing higher income group segment of the Indian population will open a potential USD 8 billion market by 2015 for selling expensive drugs. Total domestic revenues are being pegged at USD 20 billion by 2015 making India a lucrative market indeed. Health Infrastructure issues need to be dealt with in order to enable India to grab this opportunity to make itself a big consuming market for the world. Pharmacy retail is growing at the rate of 20-25 per cent annually. The organized pharma retail market size has the potential to grow to USD 9 billion by the year 2011. The size of India's pharmacy retail market is estimated at USD 4.5 billion and is dominated by 12-15 big players. Abrief historical overview of Indian industry 13
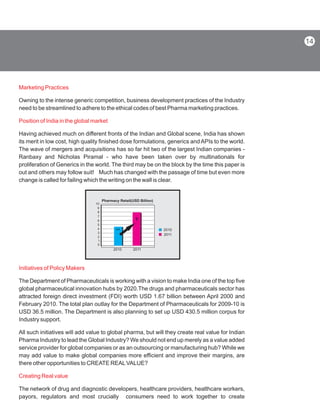
- 16. Marketing Practices Position of India in the global market Initiatives of Policy Makers Creating Real value Owning to the intense generic competition, business development practices of the Industry need to be streamlined to adhere to the ethical codes of best Pharma marketing practices. Having achieved much on different fronts of the Indian and Global scene, India has shown its merit in low cost, high quality finished dose formulations, generics and APIs to the world. The wave of mergers and acquisitions has so far hit two of the largest Indian companies - Ranbaxy and Nicholas Piramal - who have been taken over by multinationals for proliferation of Generics in the world. The third may be on the block by the time this paper is out and others may follow suit! Much has changed with the passage of time but even more change is called for failing which the writing on the wall is clear. The Department of Pharmaceuticals is working with a vision to make India one of the top five global pharmaceutical innovation hubs by 2020.The drugs and pharmaceuticals sector has attracted foreign direct investment (FDI) worth USD 1.67 billion between April 2000 and February 2010. The total plan outlay for the Department of Pharmaceuticals for 2009-10 is USD 36.5 million. The Department is also planning to set up USD 430.5 million corpus for Industry support. All such initiatives will add value to global pharma, but will they create real value for Indian Pharma Industry to lead the Global Industry? We should not end up merely as a value added service provider for global companies or as an outsourcing or manufacturing hub? While we may add value to make global companies more efficient and improve their margins, are there other opportunities to CREATE REALVALUE? The network of drug and diagnostic developers, healthcare providers, healthcare workers, payors, regulators and most crucially consumers need to work together to create 0 1 2 3 4 5 6 7 8 9 10 2010 Pharmacy Retail(USD Billion) 2011 2010 2011 4.5 9 14
- 17. something more meaningful than just improving global efficiencies. While there are different platforms and many associations, each association is rightfully working to resolve issues which their members are facing. In essence all of them are reactive and tackling current issues. While this is necessary it is also essential to think about the future. If the status quo remains and we keep focus on resolving current issues, we will not create value, we will merely survive. The risk is that the healthcare industry will grow, leaving Indian Pharma behind. Empowerment for the industry is essential and initiatives have been taken to facilitate Clinical trials, CRAMS and other services. What India needs is to find out ways and means to make India more marketable in the world. 'Made in India' label can be created in Generics,Alternate medicines andAPIs /ANIs. All stakeholders like policy makers, APIs / ANIs, manufacturers, finished dose formulations manufacturers, regulatory bodies, payors, patients and technologists, pharmacists, medical professionals will have to play their given roles besides improving their efficiency. Alternate medicines will be part of Pharma Industry and IT will further enable industry to provide virtual solutions. Payors and government will need to take responsibility towards patients. Networking of Stakeholders Nutraceuticals Formulations Patents/ Generics Funding Generics, Investment Funding Generics, Investment Medical Infrastructure API’s/ANI’s Regulation/ Policy makers Patients/ PayorsTherapies Redefinition of Global Pharmaceutical Markets 15
- 18. 4.Agenda for India Generics Alternate Medicines Global leader policy makers, industry and other stakeholders have to work in tandem to build the image of products and services offered from India. Just as Japan succeeded in shifting the perception of 'Made in Japan' from cheap to premium, India too needs to build 'Brand India' to denote superior quality at affordable prices. While on the one hand India is known for its quality and value for money in finished dose generics and APIs, on the other she is also linked with counterfeit medicines. This image needs to be wiped out in order to encash this opportunity. With its excellent re-engineering processes, better facilities, US FDA and UKMHRA approved plants and excellent quality control measures and talented professionals, India has to change its image and create high quality and low cost image of Brand India. In order to encash the next opportunity of alternate medicines, India needs to develop new molecules based on knowledge of plants. Special initiatives need to be taken up by all stakeholders to seize this opportunity. Initiatives should provide Capital, Competency and Connectivity with appropriate regulations. Global Opportunities Alternate Medicine Market Generic Market Domestic Indian Market Build “BRAND INDIA” on the basis of India’s Strength New Initiatives for the Policy Makers of India 4. AgendaforPolicymakers,IndustryandStakeholders 16
- 21. 1. The Asia Pacific Economies; CRISIL 2010 2. Current State Of Indian Economy; FICCI 2010 3. Pharmexil; Stockmarket Review,2010 4. Profile, Pharmaceutical Industry, PhRMA 2010 5. Pharma-Biotec Stock Review; Industry Outlook ,2010 6. Progress On Sanitation & Drinking Water, WHO & UNICEF 2010 7. Global Generic Pharmaceuticals Rated Universe Outlook For 2010: Patent Expirations Provide Opportunity;Analyst,2010 8. Why Generic Companies Will Dominate Future Pharmaceutical Markets; Biobusiness 2010 9. Global Pharma Looks To India: Prospects For Growth, PWC 2009 10. Exports Performance Of Indian Pharmaceutical Industry 2009, Pharmexil 11. IMS Global Pharmaceutical Forecasts;2009 12. IMS Health, Annual Report 2009 13. Pharma 2020; Challenging Business Models, PWC 2009 14. Pharma 2020; Taxing Times Ahead, PWC 2009 15. Pharma 2020; Marketing The Future, PWC 2009 16. Pharma 2020; Virtual R & D, PWC 2009 17. Pharma 2020; The Vision, PWC 2009 18. Flaming Liberal : Health care bill fails to deliver change; By Allen Acevedo;2009 19. Statistical Outline Of India 2007-2008, Tata Services Ltd. 20. India At A Glance; Development Economics LDB database; 2009 21. Go Go Generics; By Jill Wechsler; 2009 22. Health Statistics, Ministry Of Health & Family Welfare, Govt. Of India 2008 23. Impact Of Healthcare Reforms on the Healthcare Industry, LECG 2008 24. Primary Healthcare, Now More Than Ever, WHO Report 2008 25. The World Health Report, WHO 2008 26. The Institute On Global Drug Policy: Volume 2.Issue 1,The Journal Of Global Drug Policy & Practice;2008 27. India's Pharmaceutical Industry On Course For Globalization; Deutsche Bank Research,2008 28. NRHM, Framework For Implementation 2005-12, Ministry Of Health 5. References 19
- 22. & Family Welfare, Govt.Of India,New Delhi 29. Healthcare In India; Emerging Market Report PWC 2007 30. China's Generic Drugmakers Challenge India; Business Week; Sept 2007 31. Combating Generics- Pharmaceutical Brand Defense For 2007; PharmaFocus Asia 2007. 32. Critical Success Factors For The Korean Generic & API Industry In A Post FTA Environment; By David Harding; Seoul 2007 33. The Indian Pharmaceutical Industry, KPMG Report 2006 34. The Pharmaceutical industry in the Global Economy 2005;Larry Davidson & Gennadiy Greblov. 35. India-Africa-Asean & GCC Conference; Trede with Africa; Paper By Cygnus 2005 36. India At A Glance, Indiawatch 2005 37. National Health Policy-2002, Ministry Of Health & Family Welfare, Govt. Of India, New Delhi. 38. Primary Healthcare; Indian Sceario 39. Ministry Of Chemicals & Fertilizers, Govt. Of India; 40. International Conference Of Drug Regulatory Authorities;WHO http:/www.who.int/medicines/areas/quality_safety/regulation_legisla tion/icdra/en/index.htm 41. Dismantling The Gray Maze, By Agnes Shanley; 42. Addressing Regulatory Issues Through Pharmaceutical Consortia; Svetlana Lyapustina; Lee Nagoa ; Mary Devlin Capizzi; Clinical Research & Regulatory Affairs, Volume 23, Issue 1, June 2006 43. International Drug Regulation-Annual Review Of Public Health, 7(1):217 44. Healthcare Reforms (USA)(CNN); “Fox News ” 45. India's Trade Statistics & Industrial Output & Inflation Trendline; VMW Data Center 46. India's Macroeconomic Growth (GDP) ; CSO , JCIP 47. Building Japan's Generic-Drug Market, The Mckinsey quarterly, Dec 2007 48. Ensuring India's Offshoring Future, The Mckinsey Quarterly,Special Edition, 2005 49. Reports, Biogenerics 2005: A New Level Of Complexity; Datamonitor; PipelineReview.com http://pharmaceuticals.gov.in/ http://www.pharmamanufacturing.com/articles/2006/116.html 20
- 23. To make a distinctive contribution to the competitiveness, competency and performance of clients. INTERLINK – is a Business and Performance consultancy with 3 integrated portfolios spanning Business Consulting Practice, Competency Practice and Knowledge Practice, that adds value to your business through transformational insights! Our mission
- 24. Interlink Marketing Consultancy Pvt. Ltd. 3A, Central Plaza, 166 C.S.T. Road, Kalina, Santacruz (E), Mumbai - 400 098, India Tel : +91 22 2666 6611 / 44 Fax : +91 22 2666 6463 Website : http://www.interlinkconsultancy.com Insights For Business Results