Enzymes
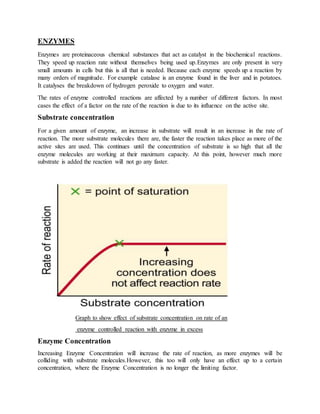
- 1. ENZYMES Enzymes are proteinaceous chemical substances that act as catalyst in the biochemical reactions. They speed up reaction rate without themselves being used up.Enzymes are only present in very small amounts in cells but this is all that is needed. Because each enzyme speeds up a reaction by many orders of magnitude. For example catalase is an enzyme found in the liver and in potatoes. It catalyses the breakdown of hydrogen peroxide to oxygen and water. The rates of enzyme controlled reactions are affected by a number of different factors. In most cases the effect of a factor on the rate of the reaction is due to its influence on the active site. Substrate concentration For a given amount of enzyme, an increase in substrate will result in an increase in the rate of reaction. The more substrate molecules there are, the faster the reaction takes place as more of the active sites are used. This continues until the concentration of substrate is so high that all the enzyme molecules are working at their maximum capacity. At this point, however much more substrate is added the reaction will not go any faster. Graph to show effect of substrate concentration on rate of an enzyme controlled reaction with enzyme in excess Enzyme Concentration Increasing Enzyme Concentration will increase the rate of reaction, as more enzymes will be colliding with substrate molecules.However, this too will only have an effect up to a certain concentration, where the Enzyme Concentration is no longer the limiting factor.
- 2. Graph to show effect of enzyme concentration on rate of an enzyme controlled reaction Temperature As the temperature rises, reacting molecules have more and more kinetic energy. This increases the chances of a successful collision and so the rate increases. There is a certain temperature at which an enzyme's catalytic activity is at its greatest. This optimal temperature is usually around human body temperature (37.5 oC) for the enzymes in human cells. Graph to show the effect of temperature on enzyme catalyzed reactions
- 3. In most chemical reactions an increase in temperature causes an increase in reaction rate. As the reactants are heated, the particles move faster and are more likely to collide with sufficient energy to overcome the activation energy of the substances reacting. Initially the rise in temperature above the optimum temperature breaks some of the hydrogen bonds that hold the shape of the active site. This reduces the efficiency of the enzyme and the reaction rate falls.If the temperature falls again the hydrogen bonds reform and the activity of the enzyme is restored. If the temperature continues to rise the disulfide bonds and covalent bonds are broken. This permanently destroys the shape of the active site and so the catalytic activity of the enzyme is lost permanently. The rise in temperature permanently denatures the enzyme. pH The pH of the environment has a major effect on the rate of enzyme controlled reactions. The intermolecular bonds – particularly the hydrogen bonds – that maintain the tertiary structure and the active site are very vulnerable to changes in hydrogen ion concentration. Each enzyme has an optimum pH at which it works at its optimum rate. A change in pH causes a change in the shape of the active site. It is not so easy for the reactants to fit in and bind to the active site. The activity of the enzyme is reduced and the rate of reaction slows. If the change is too great, the substrate will not be able to bind to the active site at all and the enzyme can no longer catalyse the reaction. In most cases the activity of the enzyme is restored as the pH returns to its optimum level. Effect of pH on rate of enzyme controlled reaction Salinity Salt concentration could affect the activities of enzymes in a couple of ways.First, salt concentration can have an effect on the equilibrium constant for the reaction being catalyzed, which does not involve the enzyme per se but will affect the outcome of the enzyme's activity.
- 4. Second, depending on how the enyzme performs its catalysis of the reaction(general acid-base, hydrogen bonding, etc), the increased salt may enhance or decrease the effect of the enzyme's chemistry. Third, some proteins are more or less stable as the salt concentration increases. Inhibition of enzyme activity Inhibitors A number of substances may cause a reduction in the rate of an enzyme catalysed reaction. Some of these (e.g. urea) are non-specific protein denaturants. Others, which generally act in a fairly specific manner, are known as inhibitors. They may be competitive or non competitive. Competitive inhibitors Competitive inhibition occurs when the substrate and a substance resembling the substrate are both added to the enzyme. In competitive inhibition, at any given moment, the enzyme may be bound to the inhibitor, the substrate, or neither, but it cannot bind both at the same time. Non-competitive inhibitors Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate.The inhibitor may bind to the enzyme whether or not the substrate has already been bound, but if it has a higher affinity for binding the enzyme in one state or the other, it is called a mixed inhibitor.