Eutrophication
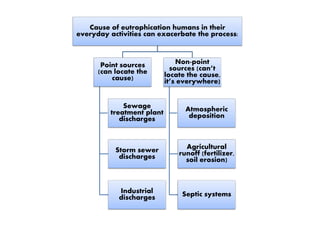
- 2. Cause of eutrophication humans in their everyday activities can exacerbate the process: Point sources (can locate the cause) Sewage treatment plant Non-point sources (can’t locate the cause, it’s everywhere) Atmospheric depositiontreatment plant discharges Storm sewer discharges Industrial discharges Atmospheric deposition Agricultural runoff (fertilizer, soil erosion) Septic systems
- 3. EUTROPHICATION It is the accumulation of nutrients in aquatic ecosystems. It alters the dynamics of a number of plant, animal and bacterial populations; thus, bringing about changes in community structure. It is a form of water pollution and like all other forms of pollution is the result of human activities influencing ecological cycles. The nutrient enrichment of an aquatic ecosystem. Natural Eutrophication -- a process that occurs as a lake or river ages over a period of hundreds or thousands of years. Cultural Eutrophication -- a process that occurs when humans release excessive amounts of nutrients; it shortens the rate ofthousands of years. Lake classification based on nutrient content and production of organic matter. Oligo- nutrient poor; meso- middle nutrient; eu- nutrient rich. it shortens the rate of aging to decades. Major Sources of Excess Nutrients Major sources of excess nutrients are agricultural fertilizers, domestic sewage and livestock wastes. 1. Agricultural fertilizers provide inorganic nutrients. 2. Sewage and wastes provide both inorganic and organic nutrients.
- 4. ADDITION OF NITRATES GROWTH OF PLANTS DEATH OF PLANTS EUTROPHICATION DEATH OF PLANTS GROWTH OF BACTERIA LACK OF OXYGEN EUTROPHICATION
- 5. fertilisers sewage (liquid domestic and industrial waste) minerals esp. nitrates minerals esp. phosphates eutrophication organicm detritus moredecomposers useupoxygen algal bloom competition for light consumers can't consume fast enough dead plants dead algae detritus organicmaterial useupoxygen byaerobicrespiration (increasedBOD) aerobesdie (invertebrates, fish,etc) anaerobicbacteria thrive.Release NH,CH,HS4 4 2
- 6. EUTROPHICATION NATURAL – LAKE CLASSIFIED BASED ON Oligo- nutrient poor; CULTURAL The addition of excess nutrients from a variety of sources results in Eutrophication brings about changes in water chemistry. These include: pH Dissolved O2 CO2 Ammonia Nitrates/Nitrites Phosphates EUTROPHICATION TYPES RESULTS poor; meso- middle nutrient; eu- nutrient rich. sources results in the rapid aging of aquatic ecosystems. During this process the species composition of the aquatic community changes. Phosphates
- 7. pH -- The pH of water reflects the CO2 contents as well as the presence of organic and inorganic acids. Values below 5 and above 9 are definitely harmful to fish and limit growth of algal and invertebrate populations. Dissolved O2 -- The amount of dissolved oxygen in water varies with temperature and pressure; high temperature or pressure, low oxygen. Most invertebrates die if oxygen levels fall below 4-5 mg/l for extended periods of time. Game fish (bass, perch, trout, etc) require oxygen to be in the range of 8-15 mg/l. CO2 -- Carbon dioxide is largely a product of aerobic and anaerobic decomposition of organic matter. It reacts with water to form carbonic acid. Normal concentrations are usually less than 1 mg/l. Fish are affected at higher levels and continued exposure to 10mg/l or more is fatal to many species.species. Phosphates -- Present in low quantities in natural waters; less than 0.01 mg/l. Released during decomposition. High levels stimulate algal blooms Ammonia (NH3 or NH4 +) -- Ammonia is a product of decomposition of animal and plant protein. It is an important plant nutrient. Natural bodies of water contain > 1 mg/l. Levels higher than this stimulate algal growth and are toxic to fish. Nitrates/Nitrites -- These N containing compounds are formed during decomposition and are inter-converted by certain species of bacteria. Natural concentrations rarely exceed 10 mg/l and are often > 1mg/l.