Glycosides
- 6. Introduction, chemistry of Cardiac glycoside Glycosides- these are organic molecules made from sugar molecule (glycone) and non sugar molecule(aglycone) witch are linked by glycosidic bond. Glycosides named specifically for the sugar contained, as glucoside (glucose), pentoside (pentose), fructoside (fructose), etc. Any of a group of organic compounds, occurring abundantly in plants, that yield a non sugar and one or more sugar substances on hydrolysis. cardiac glycoside any of a group of glycosides occurring in c ertain plants (e.g.,digitalis, Strophanthus, Urginea ),acting o n the contractile force of cardiac muscle; some are used as cardiotonics and antiarrhythmics.
- 7. Cardiotonics increase cardiac output through positive inotropic activity (an increase in the force of the contraction). They slow the conduction velocity through the atrioventricular (AV) node in the heart and decrease the heart rate through a negative chronotropic effect. general structure of a cardiac glycoside consists of a steroidal molecule attached to a sugar and an R group. The steroid nucleus consists of four fused rings to which other functional groups such as methyl, hydroxyl, and aldehyde groups can be attached to influence the overall molecule’s biological activity. different sugar groups attached at the sugar end of the steroid can alter the molecule’s solubility and kinetics; however, the lactone moiety at the R group end only serves a structural function.
- 8. Cardiac glycosides are divded as cardenolides and bufadienolides. structure of the ring attached at the R end of the molecule allows it to be classified as either a cardenolide or bufadienolide. cardenolides differ from bufadinolides due to the presence of an “enolide,” a five-membered ring with a single double bond, at the lactone end. Bufadienolides, on the other hand, contain a “dienolide,” a six- membered ring with two double bonds, at the lactone end. cardenolides are more commonly used medicinally, primarily due to the widespread availability of the plants from which they are derived. cardenolides have been primarily derived from the foxglove plants Digitalis purpurea and Digitalis lanata, while bufadienolides have been derived from the venom of the cane toad Bufo marinus, from which they receive the “bufo” portion of their name
- 9. cane toad Bufo marinus
- 11. Digitalis Purpurea, Digitalis lanata, Strrophanthius gratus and Strophanthus kombe are the major source of cardiac glycosides and digoxin, digitoxin, and ouabain (G- strophanthin) are well known cardiac glycosides.thy contain Digitoxose sugar
- 12. Digoxin is clinically used for treatment of congestive heart failure (CHF), slows the ventricular rate in Tachycardia, such as atrial fibrillation, atrial flutter, supraventricular tachycardia. Digitoxin is a cardiac glycoside. It similar in structure and effects to digoxin (though the effects are longer-lasting). Unlike digoxin (which is eliminated from the body via the kidneys), it is eliminated via the liver, so could be used in patients with poor or erratic kidney function. However, it is now rarely used in current Western medical practice. While several controlled trials have shown digoxin to be effective in a proportion of patients treated for heart failure, the evidence base for digitoxin is not as strong.
- 13. Digitoxose sugar
- 14. Cardiac glycosides exert their main pharmacological actions on the heart They increase myocardial contractility and output in a hypodynamic heart They do not cause a proportionate increase in O2 consumption Source Glycoside 1.Digitalis purpurea –purple foxglove (leaf) Digitoxin Gitoxin 2. Digitalis lanata- white foxglove (leaf) Digitoxin Digoxin 3. Strophanthus kombe (seed) Strophanthin-K 4. Strophanthus gratus (seed) Strophanthin-G
- 15. Chemistry of Cardiac Glycosides The basic chemical structure of glycosides consists of three components:- a) A sugar moiety (e.g. glucose,digitoxose) b) A steroid c) A lactone ring (5-member ring) The sugar moiety consists of unusual 1-4 linked monosaccharides. The lactone is essential for activity, the other parts of the molecule mainly determining potency and pharmacokinetic properties. Substituted lactones can retain biological activity even when the steroid moiety is removed
- 16. Introduction ,chemistry of sennosides Senna glycoside, also known as sennoside or senna, is a medication used to treat constipation Biological Source-Dried leaflets of Cassia senna (Cassia acutifolia) also known commercially as Alexandrian senna or khartoum senna and Cassia angustifolia, which is commercially known as Tinnevelly senna or Indian senna. Should not be confused with Cassia which is a common name for cinnamon. Family-Leguminosae
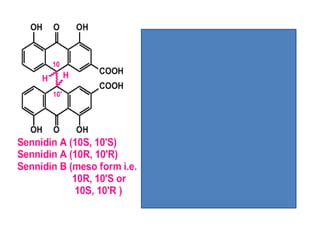
- 17. Sugar is two glucose molecules in all sennosides
- 18. Sennosides are Anthraquinones glycosides. Anthraquinones (also known as anthraquinonoids) are a class of naturally occurring phenolic compounds based on the 9,10-anthraquinone skeleton. it contains glucose as sugar part and sennidine A,B,C,D as non sugar part. 9,10-anthraquinone
- 19. Senna derivatives are a type of stimulant laxative and are of the anthraquinone type. While its mechanism of action is not entirely clear, senna is thought to act by increasing fluid secretion within and contraction of the large intestine. Administration It should be taken once daily at bedtime. Oral senna products typically produce a bowel movement in 6 to 12 hours. Rectal suppositories act within two hours. Mechanism of action The breakdown products of senna act directly as irritants on the colonic wall to induce fluid secretion and colonic motility.
- 20. Chemistry Dianthrone glycosides (1.5% – 3%), Sennosides A and B (rhein dianthrones containing the aglycon Sennidin A and Sennidin B respectively), Sennosides C and D (gylcosides of heterodianthrones rhein and aloe emodin). Senna also contain Free anthraquinones and several other glycosides such as palmidin A and aloe-emodin dianthrone diglycosides are also present. Senna also contains flavanols such as kaempferol (yellow color) and isorhamnetin. Traces of chrysophanic acid, saponin, salicylic acid and volatile oils have also been found.
- 23. Steroidal saponin glycosides- saponin have two types-1) steroidal saponin and 2)pentacyclic triterpenoid saponin Steroidal saponin are Diosgenin, Hecogenin, Sarsapogenin. They are generally used as raw material for synthesis of steroidal hormones and other steroidal compounds.
- 24. Introduction , chemistry of Diosgenin Diosgenin, a steroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins (dioscin), extracted from the tubers of Dioscorea wild yam. The sugar-free, aglycone, Diosgenin is used for the commercial synthesis of progesterone, cortisone, and other steroid products like sex hormones, oral contraceptives. The unmodified steroid has estrogenic activity and can reduce the level of serum cholesterol. Can be converted to synthetic pregnenolone and progesterone. Sources-It is present in detectable amounts in extractable amounts many species of dioscorea like dioscorea deltoidea family Dioscoreaceae,
- 27. Diosgenin content of two Indian variety viz., D. deltoidea and D. prazeri ranges from 0.32 - 1%. It is is a bioactive steroidal sapogenin belonging to the steroidal triterpene group.
- 29. Introduction, chemistry hecogenin steroid saponin isolated from Agave sisalana, family Agavaceae, Agave is one of the seventy-five varieties of general Agave family. Agave was introduced to India around 1830 by Portuguese and was used hedges along the railway track, paddy and other fields. sisal leaves are a potential source of source of hecogenin. Hecogenin is used as a base chemical in the manufacture of steroid drugs and hormones.
- 30. Agave sisalana
- 31. Hecogenin, a steroidal sapogenin is present in the leaves of many Agave plants along with tigogenin. The ratio of hecogenin to tigogenin varies considerably with the season and the age of the plant. In bulbils and young leaves of young plants, tigogenin is the predominant sapogenin, while in leaves of mature plants, hecogenin is predominant. The tigogenin yield from mature leaves is always low, regardless of origin, and is usually 0.1% of the dry weight. Hecogenin is usually high in plants of Africa and generally low in plants of other places. hecogenin was extracted from the cake obtained from the hydrolysis of sisal juice of Agave. Various organic solvents like hexane, acetone and toluene were used for extracting hecogenin.
- 32. hecogenin
- 33. steroidal hormone like cortisone can be prepared from hecogenin . The genus Agave presents sapogenin steroid- producing species, mainly hecogenin, that are important raw materials for the synthesis of steroidal drugs. Sapogenins are the aglycone (nonsugar) portions of the saponin molecule used for the semisynthesis of medicinal steroids, as corticosteroids, sexual hormones and steroid diuretics
- 34. Introduction , chemistry of Sarsasapogenin Sarsasapogenin is a steroidal sapogenin, that is the aglycon portion of a plant saponin glycosides. It is named after sarsaparilla (smilax sp.), a family of climbing plants found in subtropical regions. It was one of the first sapogenins to be identified. Sarsasapogenin is unusual in that it has a cis -linkage between rings A and B of the steroid nucleus, as opposed to the more usual trans-linkage found in other saturated steroids. Sarsasapogenin has been used as a starting material for the synthesis of other steroids. Source of sarsapogenin - Smilax ornata is a perennial, trailing vine with prickly stems. family Smilacaceae,
- 35. Smilax ornata
- 36. Sarsasapogenin is found as aglycone – in the roots of many species of monocotyledonous plant, in particular in smilax sp. sarsasapogenin saponin can be extracted from the dried powdered root with 95% ethanol. After removal of the fat from the resulting gum, the glycosidic linkage is hydrolyzed with HCL (approx. 2 M) and the resulting crude steroid is recrystallized from anhydrous acetone. The yield of pure sarsasapogenin 1.8%from Smilax root. Sarsaponin (Parillin)-(glycoside) 3 glucose, 1 rhamnose (sugar part) Sarsasapogenin (aglycon part or non sugar part)
- 38. BIOSYNTHESIS( biogenesis or anabolism) Biosynthesis is multistep enzymes catalyzed reaction in witch two or more smaller or simpler molecule join together to form larger or complex molecules in living organisms. this process consist of various metabolic pathways. Metabolic pathway completed in presence of enzymes
- 39. Living plants are biosynthetic laboratories for synthesis of primary and secondary metabolites. Photosynthesis reaction involve use of carbon dioxide , water and light to form glucose molecule.
- 42. Biosynthesis of glycosides It involves following two steps 1) transfer of uridylyl group from uridyl triphodphate (UTP) to sugar-1-phosphate forming UDP-sugar and inorganic pyrophosphate. enzyme uridylyl transferase catalyse this step. UTP+sugar-1-phosphate= UDP-sugar + pyrophosphate 2) transfer of sugar from UDP to suitable acceptor (aglycon), mediated by enzyme glycosyl transferase, thus forming glycoside UDP-sugar +acceptor(aglycon)= aglycon-sugar(glycoside)+ UDP
- 43. Biosynthesise of digoxin & digitoxin
- 44. Biosynthesis of sennosides anthraqunone aglycone are biosynthesised from acetate mevalonate pathway via polyketide formation. about eight acetate units combine to form polyketide
- 46. sennosides
- 48. Beta sitosterol