Good Documentation Practices
- 2. WHAT ARE GOOD DOCUMENTATION The Definition of Good Documentation Practice (GDP) Describes Standards By Which Documentation Is Created And Maintained In The Pharmaceutical Industry. WHY GDP IS SO IMPORTANT? • Regulatory Requirement • Customers requirement • Documentation error can lead to severe consequence – Product safety concerns – Litigations (Law suits) – Action by the regulators
- 3. GDP IS AN ADVERTISEMENT Rs 35-40 Crores So in the same way, Document is our advertisement to show How good we are. How committed we are How sincere we are
- 4. Different types of documents • For organization & personnel • For building & facilities • For equipment's • For production & process control • For handling of material • For packing and labelling control. • For holding and distribution • For laboratory control • For records and reports • For return goods
- 5. Documentation standards – PERMANENT – LEGIBLE – ACCURATE – PROMPT – CLEAR / CONCISE – CONSISTENT – COMPLETE – DIRECT – TRUITHFUL – CURRENT – TRACEABLE / ATTRIBUTABLE
- 6. Documentation standard - PERMANENT – Information can’t be changed, erased or washed off. Proper writing instrument No pencil No felt-tip pens Only ball point pens Permanent Ink. Black or Blue
- 7. Documentation standard - LEGIBLE Correct errors properly Draw a single line through the in-correct entry Make the correct entry adjacent to the incorrect entry In-correct entry must still be legible through the cross-out. Sign & date the correction Date of correction is the date , the correction was made, not the date the error was made. Information can be easily read
- 8. Documentation standard - LEGIBLE Do not Scribble out or black out with pen Write over White out Information can be easily read
- 9. Documentation standard - LEGIBLE Information can be easily read
- 10. Documentation standard - ACCURATE Information recorded is accurate and calculations are correct Lot numbers, Serial numbers and product codes are double checked. Perform calculations at least twice Have the calculations reviewed and verified by second person. Present results in units specified. Check spelling in product names Compare hard copy vs Electronic copy.
- 11. Documentation standard - PROMPT Information recorded in timely manner Document tasks immediately after they are performed Never predate Never post date If you missed a step be truthful. Document it. And complete it at that time if possible noting the actual date it was completed.
- 12. Documentation standard - CLEAR Every one who reads the document has the same understanding of what it means Looking at document five years from now , would some one understand what I wrote. If you missed a step be truthful. Document it. And complete it at that time if possible noting the actual date it was completed. Areas identified as not applicable shall be close with –N/A- with sign & date.
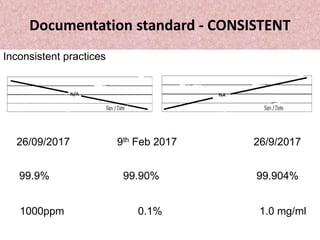
- 13. Documentation standard - CONSISTENT Information such as dates, times, and abbreviations are recorded to company standards. Practices should be consistent among all employees Every form must be filled in the same way every time. Date format should be consistent Time format should be consistent Reporting decimals should be consistent Measuring units should be consistent
- 14. Documentation standard - CONSISTENT Inconsistent practices 26/09/2017 9th Feb 2017 26/9/2017 99.9% 99.90% 99.904% 1000ppm 0.1% 1.0 mg/ml
- 15. Documentation standard - COMPLETE All information included All required data filled correctly No blank spaces, Pages, or portion of the page. N/A can only be used when it is very clear that a portion of document , form , record does not apply. All signatures available with dates. All supporting data attached.
- 16. Documentation standard - DIRECT Information is recorded directly on to the proper form, Laboratory note book or computer system. Original entry should made directly on to the GMP document. No scrap paper or Sticky notes. Do not write on palms, walls, Tables Gloves and containers
- 17. Documentation standard - TRUITHFUL All Information included in the document is, to the writers knowledge and ability is factual. Your signature says information is true. Information must never be falsified. Because you are certifying by signature.
- 18. Documentation standard - CURRENT All documents are of current, All formats are of current Check current version every time before use Check approved version every time before use
- 19. Documentation standard - TRACEABLE All documents should be traceable / Attributable Make it clear who logged the information What it was, and when and why it was documented.
- 20. GDP STEPS. Signature & date: All entries in a GMP document must be accompanied by the identity of person and date that the entry was made. Recording time: Recording time in military format for each activity. Two digits two indicate Hours and Two digits to indicate minutes. (08:15 14:05) Corrections: No hand written changes or Corrections allowed on printed text or an approved GMP document. Corrections in handwritten entries should done as detailed above.
- 21. GDP STEPS. Performed by: Performance should be documented after completion of step and before moving on to next step. Do not perform a step if procedure not available Recorded by: Recorded by is used if the operator performing the step and unable to record for any specific reason, Data must then be recorded by another person watching the operation with signature and date. Verified by Verification activity shall be done before moving on to next step. Verification can not be done for their own activities Verification shall be done by second person who witnessed the activity and proficient in the task. Approved by Signature of qualified individual indicating that the documented information is complete, accurate and acceptable.
- 22. GENERAL REQUIREMENTS OF GDP Clearly written documents / Using words that can every one can understand Using indelible ink Legible handwritten entries Performed by signatures Reviewing and approving Signed delegation of responsibility Page numbering Providing enough details to make sense of it in the future. Consistent wording to avoid confusion