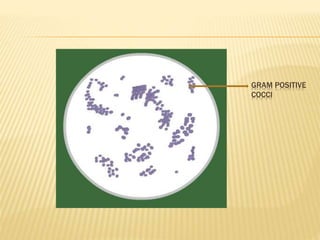
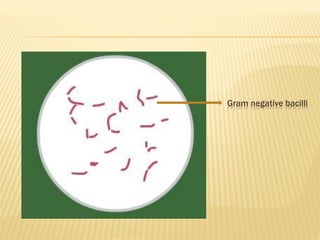
Gram stain
- 2. TOPICS STAINS GRAM STAIN MECHANISM OF GRAM STAIN PREPARATION OF STAINS MODIFICATION OF STAINS PROCEDURE OF SMEAR PREPARATION & GRAM STAINING COMMON ERROR WHILE STAINING LIMITATION OF GRAM STAIN :
- 3. STAINS Stains are natural or synthetic substances that render the structure of cell visible. Live bacteria do not show much structural details under the light microscope due to lack of contrast. So it is customary to use staining technique to produce colour contrast.
- 4. IMPORTANCE OF STAIN: The microscopical examination of stained smear enables the morphology, relative sizing and arrangement of microorganism to be seen clearly. It also assist in the detection of cells, esp.pus cells, PMN cells. Bacteria can also be differentiated by their staining reaction. e.g. gram positive from gram negative organisms.
- 5. TYPES OF STAINS: Simple staining Eg: methylene blue, basic fuchsin Negative eg: india ink,nigrosin Metachromatic eg: alberts, ponders Differential eg:grams, acid fast Impregnation eg: silver impregnation Fluorescent stain eg:acridine orange, rhodamine auramine, calcoflour white
- 6. GRAM STAIN This is the most important staining method in bacteriology. It was originally devised by the histologist Christian Gram in 1884 as a method of staining bacteria in tissues.
- 7. The Gram staining requires the application of four reagents in the following order: PRIMARY STAINING with basic pararosaniline dye, such as crystal violet, methyl violet or gentian violet.. Application of a dilute solution of IODINE. DECOLOURISATION with an organic solvent such as ethanol ,acetone or aniline. COUNTERSTAINING with a dye of contrasting colour, such as carbol fuchsin ,safranine or neutral red.
- 8. The violet dye and iodine combine to form an insoluble , dark purple compound in the bacterial protoplasm and cell wall. This dye – iodine complex is dissociable in the decolourizing agent, which dissolves and removes this two components from the cell. Bacteria which retain this complex stain as dark purple and bacteria which are decolorized take the counter stain and appear pink. Bacteria which stain dark purple are called Gram positive and which stain pink are called Gram negative.
- 9. The exact mechanism of Gram reaction is not understood. Various theories that have been suggested are as follows: Gram positive cells have a more acidic protoplasm, which may account for their retaining the basic primary dye more strongly than Gram negative bacteria. The peptidoglycan of Gram positive bacterial cell wall is thicker and denser than Gram negative thus able to retain dye iodine complex. The high lipid content of Gram negative bacteria makes them permeable to secondary dye after
- 12. Gram positive cocci in pair and chain
- 13. PREPARATION OF STAIN crystal violet: crystal violet 10 gm 100% Ethanol(absolute alcohol) 100ml distilled water 1000 ml Crystal violet is used at concentration of 0.5-2% solution. This solution keeps well and is perfectly satisfactory for general use. However , Gram positive staining can be strengthened by addition of sodium bicarbonate or ammonium
- 14. Kopeloff & Beerman STAIN: -Solution A - Methyl violet 10g -Distilled water 1 L Solution B -Sodium bicarbonate 50g -Distilled water 1 L - Shortly before use, mix 30 volumes of sol. A to 8 volumes of sol.B This mixture precipitate within few days so can't store for long time.
- 15. Preston & Morrell’s stain: Crystal violet 20g Methylated spirit 200ml Ammonium oxalate, 1% in water 800ml
- 16. IODINE SOLUTION: Gram’s (lugol’s iodine)solution: iodine crystals 10gm potassium iodide 20gm distilled water 1000ml Koepleoff & Beerman’s iodine - Iodine 200gm - Sod.Hydroxide 1mol/L 100ml - Distilled water 900ml
- 17. DECOLORIZERS Fastest- acetone(2-3 sec) Useful when only single slide is to be stained. However, short period of exposure is difficult to control when many slide has to be stain. Slowest-ethanol,95%(1 min or more) This act more slowly. So it should be applied and reapplied for about 1 min. Intermediate-acetone-alcohol(10 sec.) ethanol,95% 100ml Acetone 100ml Combine in brown colored bottle
- 18. Preston & Morrell have shown that addition of a small concentration of iodine to acetone slows the rate of decolourazation without reducing its specificity. The decolorizer made are as follows: LIQUOR IODI FORTIS Iodine 10gm Methylated spirit 6gm Distilled water 10ml IODINE-ACETONE Liquor iodi fortis 35ml Acetone 965ml
- 19. COUNTER STAINS Dilute carbol fuchsin: Strongest red staining within 10-30 sec. Colouration is so dark that sometime difficult to distinguish Gram negative from Gram positive bacteria. Basic fuchsin : Applied for 10-30 sec. This is recommended for general use. Basic fuchsin 0.5 gm Distilled water 1000ml Safranine: Used as 0.5% in distilled water.
- 20. MODIFICATION OF GRAM’S STAIN KOPELOFF AND BEERMAN'S MODIFICATION: Primary stain solution consists of freshly constituted methyl violet with sodium bicarbonate in distilled water. Mordant consists of iodine dissolved in 4% NaOH solution. Decolonization is either using acetone alone or a mixture of acetone and ethanol. Basic fuchsin is used to counter stain the smear. This method may be modified to stain tissue
- 21. KOPELOFF & BEERMAN’S STAIN FOR SECTION: It is same as smear stain method of kopeloff & Beerman’s with addition steps as follow: After counterstaining ,dehydrate quickly the slide by flooding with 95% alcohol followed by absolute alcohol only for brief period of time. End this contact by immersing the slide in xylene.
- 22. JENSEN'S MODIFICATION This method involves use of methyl violet as primary stain, iodine and potassium iodide in water as mordant, absolute alcohol as decolorizer and neutral red as counter stain. For Neisseria spp
- 23. PRESTON AND MORRELL'S MODIFICATION The primary stain used in this modification is ammonium oxalate-crystal violet (30 sec).The smear is washed in Lugol's iodine and further treated with iodine solution(30 sec). The smear is decolorized using iodine-acetone decolorizer (30 sec)and counterstained using dilute carbol fuchsin solution(30 sec). This method has been further modified to overcome the irritating iodine in aerosols by reducing the iodine concentration to one-tenth and shortening the duration of decolonization to ten seconds.
- 24. PREPARATION OF SMEAR AND GRAM STAIN PROCEDURE PREPARATION OF SMEAR: Smear should be prepare evenly covering an area of about 15-20mm diameter on the slide. From purulent specimen: Take loopful specimen with sterile inoculating wire From nonpurulent specimen: Centrifuge and then make smear From sputum: Take purulent or caseous material
- 25. From swab: Roll the swab on slide From culture plate: Put a drop of saline on a clean slide .with the help of sterile inoculating wire ,individual colony is picked up and mix with saline. LABELLING OF SLIDE: With diamond or grease pencil. Stick on paper should not be used.
- 26. FIXATION OF SMEAR: It is to preserve the smear being washed of from slide while staining. Heat fixation: After air drying of smear is properly done,pass the slide rapidly three times through blue flame of Bunsen burner. Disadvantage: Excessive heating damage the organism and alter the staining reaction. It also damage leucocytes. So not suitable for intracellular organism like N.gonorrheria & N.meningitidies Alcohol fixation:
- 27. OTHER CHEMICAL FIXATIVES: Potassium permanganate(anthrax bacilli) Formaldehyde vapor (mycobacterial species)
- 28. PROCEDURE: Lay the fixed slide on a staining rack. Cover the smear completely with gential violet. keep it for 1 min. Wash under running tap water & flood the slide with Gram’s iodine solution and wait for 1 min. Gently drain off the iodine solution and rinse with running tape water. Decolorize rapidly (few seconds) with acetone/alcohol . Wash the slide under running tap water . Pour safranin solution on the slide and keep it for 30 seconds. Wash off the stain with tap water. Wipe the back of the slide clean. Place in a draining rack for the smear to air dry. Examine the smear microscopically with oil immersion objective.
- 29. In Gram staining gentian violet, Gram’s iodine,saffranin are kept for 1 min and discoloration is rapid within few seconds. Rapid Gram stain refers to quickened technique where the smear is exposed to only 30 seconds instead of one minute. Used when only single slide has to be stain.
- 32. LIMITATIONS OF GRAM STAINING: Some Gram-positive bacteria may lose the stain easily and therefore appear as a mixture of Gram-positive and Gram-negative bacteria (Gram-variable). When over-decolorized, even Gram positive bacteria may appear pink and when under-decolorized gram negative bacteria may appear Gram positive. Small and slender bacteria such as Treponema, Chlamydia, Rickettsia are often difficult to stain by Gram's method
- 33. Gram positive cells affected by cell wall active agents such as lysozyme or antibiotics may become Gram negative. Bacteria totally devoid of cell wall (Mycoplasma) are always Gram negative
- 34. THANK YOU