hplc2.pptjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjj
- 1. الكروماتوغر و الكيميائية الفصل طرق افية 458 كيم Chemical Separation and Chromatographic Methods Chem 458
- 2. High Performance Liquid Chromatography (HPLC)
- 3. Liquid chromatography It is the first ever described chromatographic method (by Tswett in 1903) Unlike gas chromatography, the sample in liquid chromatography must not be vaporized so, almost all kinds of compounds can be analysed by liquid chromatography The development of instrumental liquid chromatography was later than for gas chromatography because of the higher pressure needed for the former HPLC is considered to be the major chromatographic technique available today for non-volatile or heat-sensitive substances.
- 4. A little history: The official date of birth of chromatography is the 21 March 1903 in Warsaw when Mikhail Semenovitch TSWETT has presented at the Congress of the Polish Natural Sciences Society a communication entitled: « A new class of adsorption phenomena and their applications in biochemical analysis » about the separation and purification of vegetal pigments (a mixture of chlorophylls and xantophylls) on a chalk column plant extract in solvent
- 5. A little history: 1938 : REICHSTEIN proposes a theory for the elution and separation of solutes on a column 1952 : application of gradient elution 1967 : beginning of HPLC after the works of HUBER and HUZSMAN, this technique was first named « High Speed Liquid Chromatography » then « High Pressure Liquid Chromatography » and finally « High Performance Liquid Chromatography » 1969 : after the 5th International Symposium International « Advances in Chromatography » the development of HPLC was very fast The term HPLC is appropriate for separations of any size (from micro-analytical to preparative) if the particles of the stationary phase are not larger than about 10µm
- 6. Fundamental definitions (to IUPAC nomenclature) Chromatography: a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while the other (the mobile phase) moves in a definite direction Chromatogram: a graphical or other presentation of detector response, concentration of analyte in the effluent or other quantity used as a measure of effluent concentration versus effluent volume or time Stationary Phase: one of the two phases forming a chromatographic system. It may be a solid, a gel or a liquid. If a liquid, it may be distributed on a solid. This solid may or may not contribute to the separation process. The liquid may also be chemically bonded to the solid (bonded phase: covalently bonded to the support particles or to the inside wall of the column tubing) or immobilized onto it (immmobilized phase) Mobile Phase: a fluid which percolates through or along the stationary bed, in a definite direction. It may be a liquid (liquid chromatography) or a gas (gas chromatography) or a supercritical fluid (supercritical-fluid chromatography)
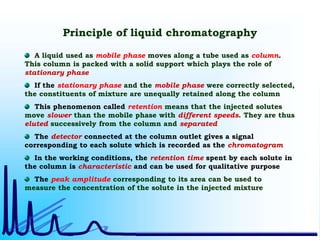
- 7. Principle of liquid chromatography A liquid used as mobile phase moves along a tube used as column. This column is packed with a solid support which plays the role of stationary phase If the stationary phase and the mobile phase were correctly selected, the constituents of mixture are unequally retained along the column This phenomenon called retention means that the injected solutes move slower than the mobile phase with different speeds. They are thus eluted successively from the column and separated The detector connected at the column outlet gives a signal corresponding to each solute which is recorded as the chromatogram In the working conditions, the retention time spent by each solute in the column is characteristic and can be used for qualitative purpose The peak amplitude corresponding to its area can be used to measure the concentration of the solute in the injected mixture
- 8. Simplified scheme of the chromatographic separation process t = 5mn t = 10mn most least interactions with stationary phase injector detector flow of mobile phase t = 0 column
- 9. Main modes in liquid chromatography There are several modes in high performance liquid chromatography They are classified according to the mechanism of separation Each mode corresponds to a given kind of interaction: surface adsorption solvent partitioning ion exchange size exclusion
- 10. Adsorption chromatography the stationary phase is a solid adsorbant retention is due to a series of adsorption / desorption steps separation is based mainly on differences between the adsorption affinities of the sample components for the surface of the active solid (liquid solid chromatography)
- 11. Adsorption chromatography silica and alumina are the most used stationary phases both solute and solvent can be attracted by the active sites at the surface of the stationary phase the molecules are retained by the interaction of their polar functional groups with the surface functional groups such as silanols of silica if solutes have different interactions with the adsorbing sites the separation can occur Si O Si O Si O O O H H H Silanol groups Si-OH at the surface of silica
- 12. Partition chromatography the stationary phase is a liquid coated or linked to a solid support retention is due to the partitioning of the solute between the two liquid phases (relative solubility) separation is based mainly on differences between the solubilities of the components in the mobile and stationary phases (liquid liquid chromatography)
- 13. Partition chromatography the most retained species is that having the highest affinity (solubility) for the liquid stationary phase, relatively to the mobile phase (eluent) separation is based on the differences in relative solubility There are two modes in liquid chromatography « normal » mode : polar stationary phase and non-polar mobile phase (the first mode described). In this procedure, the stationary phase is more polar than the mobile phase. This term is used in liquid chromatography to emphasize the contrast to reversed-phase chromatography « reversed-phase » mode: non-polar stationary phase and polar mobile phase (the most used mode). In this procedure the mobile phase is significantly more polar than the stationary phase, e.g., a microporous silica-based material with chemically bonded alkyl chains
- 14. Ion exchange chromatography (IEC) the stationary phase has ionically charged groups at the surface the retention is due to the attractive interactions between ionic solutes and the opposite charged stationary phase separation is based mainly on differences in the ion exchange affinities of the sample components this technique is now often referred to as Ion Chromatography (IC)
- 15. Size exclusion chromatography (SEC) the stationary phase is a porous material having controlled pore size separation is based mainly on exclusion effects, such as differences in molecular size and/or shape the terms Gel Filtration and Gel-Permeation Chromatography (GPC) were used earlier to describe this process
- 16. Size exclusion chromatography (SEC) In this mode, each column can separate solutes having specific size range separation mechanism is sieving the larger species cannot enter all the pores and will elute first because they have a shorter path in the column this mode is very useful for the determination of molecular size of macromolecules (polymers, proteins,…) large molecules excluded from pores - not retained, first eluted intermediate molecules: retained, intermediate elution times small molecules permeate into pores: strongly retained, last eluted
- 17. Importance of polarity in HPLC the notion of polarity plays a fundamental role in HPLC all chemicals have a unique and characteristic behaviour related to their molecular structure and electron charge distribution they can be described as being “ polar ” or “ non-polar ”, with a range of polarities between the most polar and most non-polar water is a good example of a very polar liquid, and paraffin based oil is a good example of a very non-polar liquid this “ polarity ” characteristic of chemicals allows to explain the chromatographic “ retention mechanisms ” that are used to create many HPLC separations a simple rule describes this behavior for polarity-based retention mechanisms: “Like Attracts Like, and Opposites are Not Attracted”
- 18. Mobile phase composition: isocratic analysis: in this procedure the composition of the mobile phase remains constant during the elution process gradient elution: in this procedure the composition of the mobile phase is changed continuously or stepwise during the elution process
- 19. Elution process: During the solute transfer in the column, it shows a typical broadening due to the diffusion phenomena (transversal and longitudinal) The solute band width increases with the retention time giving a typical peak broadening
- 20. The chromatogram: characteristic parameters tM : dead time (for a « non-retained solute ») tR : retention time, characteristic of each solute
- 21. Some fundamental equations: Corrected retention time: t’R = tR – tM average linear velocity (ū) is measured from the retention time of an unretained substance (tM) which moves at the same velocity as the mobile phase: ū = L / tM Retention factor (or capacity ratio) k : corresponds to a relative retention: k = t’R / tM = (tR – tM) / tM since: tM = L / ū we can write: tR = (1 + k) . tM = (1 + k) . L / ū Hence the retention time is directly proportional to the column length L and inversely proportional to the linear flow rate of the mobile phase ū when k is ≤ 1.0, separation is poor when k is > 30, separation is slow when k is 2-10, separation is optimum
- 22. Some fundamental equations: average linear velocity (ū) is measured from the retention time of an unretained substance (tM) which moves at the same velocity as the mobile phase: ū = L / tM = F / q where: F is the mobile phase flowrate and q the free cross-sectional area of the column the column porosity eT is: eT = q / p . r2 = F . tM / p . r2 . L = F . tM / VR where: r is the radius of the column and VR the volume of the empty column
- 23. Column efficiency: The chromatographic peaks being supposed gaussian, the peak broadening can be related to the separation and the column efficiency which is evaluated by the number of theoretical plates of the column N (similarly to distillation process) which is a number indicative of column performance. For a gaussian peak, N can be calculated by one of the following equations: N = (tR / s)2 (s: standard deviation of the peak) N = 16 (tR / w)2 (w: width at baseline) N = 5,54 (tR / d)2 (d: width at half- height) In order to compare columns having different lengths, one calculate the plate height or height equivalent to a theoretical plate HETP: H = L/N (L : column length) H may vary from centimeters (packed columns) to several microns (high resolution capillary columns)
- 24. Column efficiency: Column selectivity: a = t’R2 / t’R1 = k2 / k1 ( separation occurs only if a > 1 ) Resolution between two neighboring peaks: RS = 2 (tR2 – tR1) / (w2 + w1) = 1,18 (tR2 – tR1) / (d2 + d1) For two neighboring peaks, a resolution RS higher than 1 means a complete separation (for RS = 1, the overlapping peak surface is 2%) When RS is less than 0.8, the separation between the two peaks is considered to be incomplete
- 25. Optimization of column efficiency and resolution Resolution (and zone broadening) depends on: ū (linear flow rate): low flow favors increased resolution H (plate height) (or N number of plates): use smaller particles, lengthen column, viscosity of mobile phase (diffusion) a (selectivity factor): vary temperature, composition of column/mobile phase k (capacity factor): vary temperature, composition of column/mobile phase time detector signal A B A B A B tM tRA tRB RS = 0.75 RS = 1.0 RS = 1.5
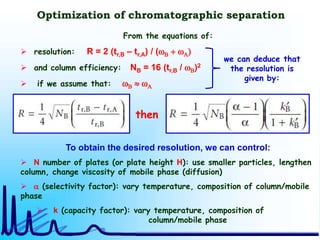
- 26. Optimization of chromatographic separation then From the equations of: resolution: R = 2 (tr,B – tr,A) / (wB + wA) and column efficiency: NB = 16 (tr,B / wB)2 if we assume that: wB wA To obtain the desired resolution, we can control: N number of plates (or plate height H): use smaller particles, lengthen column, change viscosity of mobile phase (diffusion) a (selectivity factor): vary temperature, composition of column/mobile phase k (capacity factor): vary temperature, composition of column/mobile phase we can deduce that the resolution is given by:
- 27. HPLC equipment
- 28. Main parts of HPLC equipment a b d c e f g h a- solvent reservoirs e- precolumn and column b- gradient elution controller f- detector c- pumping system g- data processing d- sample injection h- solvent waste
- 29. Agilent HPLC equipment mobile phase storage filtration and degassing preparation of eluent mixtures HPLC pump autosampler column box and oven control unit injection valve detection unit with UV-visible lamp
- 30. High performance liquid chromatography (HPLC) animation https://www.youtube.com/watch?v=ZN7euA1fS4Y
- 31. The mobile phase In HPLC the quality of the mobile phase is crucial The solvent is either water (preferred) or organic All solvents should be of ‘HPLC grade’ This means: a high purity a prior filtration on a 0.2µm filter The ‘HPLC grade’ solvent can be either purchased or produced using a convenient filtration device Using filtered and pure solvents avoids many problems with pumps and columns and also improves the quality of analyses All solvents should be degassed prior to use in HPLC
- 32. Reciprocating piston pump It is the most widespread pumping system used in HPLC: a- motor b- transmission c- tightness seal d- piston e- solvent inlet f- inlet and outlet checkvalves g- solvent outlet a g f e d c b
- 33. Injection system in HPLC The best injection device uses a switching six-port valve with a sampling loop This system allows an easy and reproducible injection of liquid samples It operates in two steps: load and inject « load » position sample sample mobile phase mobile phase column column outlet outlet Sample loop « inject » position
- 34. The HPLC column Most stationary phases are chemically bonded to the solid support which means a greater stability The stationary phase parameters are accurately controlled to assure constant and reproducible performances Packing techniques have also improved in order to obtain smaller but more efficient columns A typical HPLC column End fittings
- 35. Detection in HPLC HPLC detectors are commonly classified as: general (or bulk property detector): it measures the difference in some physical property between the chromatographic effluent and the pure mobile phase. This kind of detector, such as refractive index detector, is considered as universal and has a general use but a low sensitivity and a limited dynamic range specific (or solute property detector): responds to a physical or chemical property characteristic of the solute. It provides high sensitivity with a wide linear range such as in spectrophotometric detectors. Although this kind of detectors is specific it is more widely used than bulk property ones
- 36. Spectrophotometric UV/visible detector It is a simple and cheap detector and the most widely used detector in HPLC It is a solute property detector It is specific to solutes which exhibit an absorption in the UV/visible range (generally unsaturated compounds) The mobile phase must not significantly absorb at the measured wavelength With favourable solutes, the sensitivity (minimum detectable concentration) is about 5.10-8g.mL-1
- 37. UV detector (with photodiode array ) photodiode array sample cell deuterium lamp holographic grating quartz lens