IITJEE -Chemistry 2011-i
- 1. IIT JEE –Past papersCHEMISTRY- UNSOLVED PAPER - 2011
- 2. SECTION – ISingle Correct Answer TypeThis section contains 7 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct.
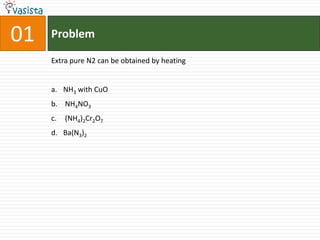
- 3. 01ProblemExtra pure N2 can be obtained by heatingNH3 with CuO NH4NO3 (NH4)2Cr2O7Ba(N3)2
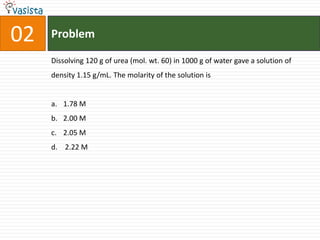
- 4. Problem02Dissolving 120 g of urea (mol. wt. 60) in 1000 g of water gave a solution of density 1.15 g/mL. The molarity of the solution is1.78 M 2.00 M2.05 M 2.22 M
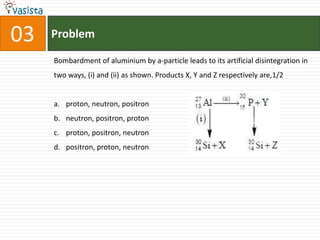
- 5. Problem03Bombardment of aluminium by a-particle leads to its artificial disintegration in two ways, (i) and (ii) as shown. Products X, Y and Z respectively are,1/2proton, neutron, positron neutron, positron, protonproton, positron, neutron positron, proton, neutron
- 6. Problem04Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, areoctahedral, tetrahedral and square planar tetrahedral, square planar and octahedralsquare planar, tetrahedral and octahedral octahedral, square planar and octahedral
- 7. FOR SOLUTION VISIT WWW.VASISTA.NET
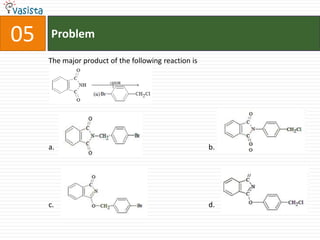
- 8. Problem05The major product of the following reaction is b.c. d.
- 9. Problem06Among the following compounds, the most acidic isp-nitrophenolp-hydroxybenzoic acido-hydroxybenzoic acid p-toluic acid
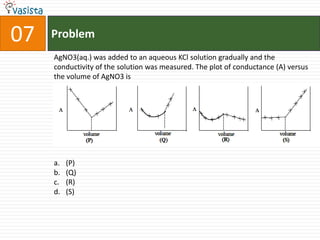
- 10. Problem07AgNO3(aq.) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance (A) versus the volume of AgNO3 is(P) (Q)(R) (S)
- 11. SECTION – IIMultiple Answer TypeThis section contains 4 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which ONE OR MORE may be correct.
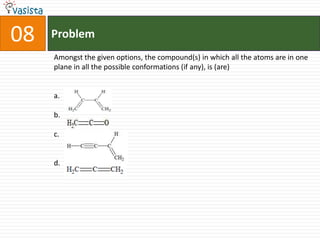
- 12. 08ProblemAmongst the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (if any), is (are)a.b.c.d.
- 13. Problem09Extraction of metal from the ore cassiteriteinvolvescarbon reduction of an oxide ore self-reduction of a sulphide oreremoval of copper impurity removal of iron impurity
- 14. Problem10According to kinetic theory of gasescollisions are always elasticheavier molecules transfer more momentum to the wall of the containeronly a small number of molecules have very high velocitybetween collisions, the molecules move in straight lines with constant velocities
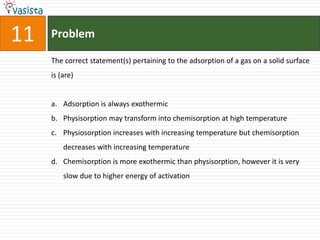
- 15. Problem11The correct statement(s) pertaining to the adsorption of a gas on a solid surface is (are)Adsorption is always exothermicPhysisorption may transform into chemisorption at high temperaturePhysiosorption increases with increasing temperature but chemisorption decreases with increasing temperatureChemisorption is more exothermic than physisorption, however it is very slow due to higher energy of activation
- 16. SECTION – IIIParagraph TypeThis section contains 2 paragraphs. Based upon one of paragraphs 2 multiple choice questions and based on the other paragraph 3 multiple choice questions have to be answered. Each of these questions has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct.
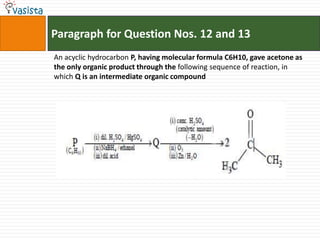
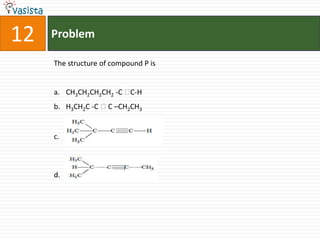
- 17. Paragraph for Question Nos. 12 and 13An acyclic hydrocarbon P, having molecular formula C6H10, gave acetone as the only organic product through the following sequence of reaction, in which Q is an intermediate organic compound
- 18. 12ProblemThe structure of compound P isCH3CH2CH2CH2 -C C-HH3CH2C -C C –CH2CH3..
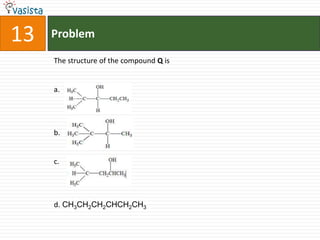
- 19. Problem13The structure of the compound Q isa.b.c.d. CH3CH2CH2CHCH2CH3
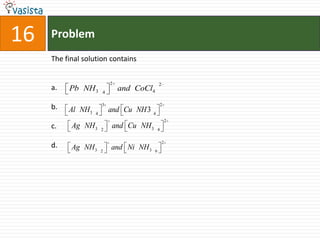
- 20. Paragraph for Question Nos. 14 to 16When a metal rod M is dipped into an aqueous colourless concentrated solution of compound N, the solution turns light blue. Addition of aqueous NaCl to the blue solution gives a white precipitate O. Addition of aqueous NH3 dissolves O and gives an intense blue solution.
- 21. Problem14The metal rod M isFe CuNi Co
- 22. Problem15The compound N isAgNO3Zn(NO3)2Al(NO3) 3Pb(NO3) 2
- 23. Problem16The final solution containsa.b.c.d.
- 24. SECTION – IVInteger Answer TypeThis section contains 7 questions. The answer to each of the questions is a single digit integer, ranging from 0 to 9. The bubble corresponding to the correct is to be darkened in the ORS.
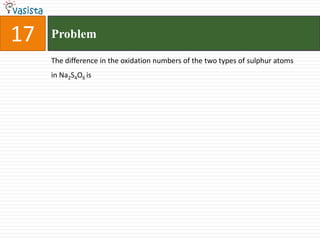
- 25. 17ProblemThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is
- 26. Problem18A decapeptide (Mol. Wt. 796) on complete hydrolysis gives glycine (Mol. Wt. 75), alanine and phenylalanine. Glycine contributes 47.0% to the total weight of the hydrolysed products. The number of glycine units present in the decapeptide is
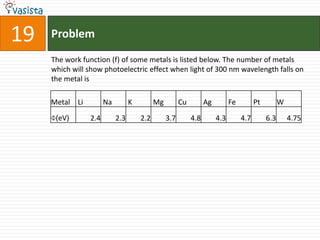
- 27. Problem19The work function (f) of some metals is listed below. The number of metals which will show photoelectric effect when light of 300 nm wavelength falls on the metal is
- 28. Problem20The maximum number of electrons that can have principal quantum number, n = 3, and spin quantum number, ms = - will be
- 29. Problem21Reaction of Br2 with Na2CO3 in aqueous solution gives sodium bromide and sodium bromate with evolution of CO2 gas. The number of sodium bromide molecules involved in the balanced chemical equation is
- 30. Problem22To an evacuated vessel with movable piston under external pressure of 1 atm, 0.1 mol of He and 1.0 mol of an unknown compound (vapour pressure 0.68 atm. at 0oC) are introduced. Considering the ideal gas behaviour, the total volume (in litre) of the gases at 0oC is close to
- 31. Problem23The total number of alkenes possible by dehydrobromination of 3-bromo-3-cyclopentylhexane using alcoholic KOH is
Editor's Notes
- E