Lab component of dx tb
- 1. Laboratory Diagnosis of TB • Presenter: WATI VUKIALAU-VULAVA LT/NTP - NORTH
- 2. Objectives 1. 2. 3. 4. 5. 6. 7. History of TB elements of DOTS What the bacteria looks like Lab component of diagnosing TB Sputum collection procedures What good and poor sputum quality is Available Lab tests in Fiji to detect TB
- 3. History of TB • TB has affected humans for millennia • Historically known by a variety of names, including: – Consumption – Wasting disease – White plague • TB was a death sentence for many
- 4. Cont…History of TB Scientific Discoveries in 1800s • Until mid-1800s, many believed TB was hereditary • 1865 Jean AntoineVillemin proved TB was contagious • 1882 Robert Koch discovered M. tuberculosis, the bacterium that causes TB Mycobacterium tuberculosis Image credit: Janice Haney Carr
- 5. Elements of DOTS Strategy • Political commitment to TB control • Case detection by sputum smear microscopy • Regular, uninterrupted supply of high quality anti-TB drugs • Directly Observed Treatment (DOT) • Standardised recording and reporting
- 6. Mycobacterium tuberculosis * long, slender, straight or curved, acid fast bacilli * slow growers, obligate aerobes, intracellular bacterium * structure composed of high molecular weight acidic waxes, mycolic acid, cord factor
- 7. WHY DO WE COLLECT SPUTUM? 1. To detect & diagnose TB (mycobacterium TB)
- 8. •Smear-positive patients are 4-20 times more infectious •Untreated, a smear-positive patient may infect 10-15 persons/year •Smear-positive patients are much more likely to die if untreated
- 9. 2. To monitor effectiveness of treatment
- 10. When is the best time?? • The best time, but not the only time to collect a sputum specimen is upon awakening in the morning • When more than 1 specimen is required they will be collected on different days • Any specimens collected in one day will count as the first (1) specimen
- 11. Sputum Collection • 3 specimens considered
- 12. Cumulative Positivity 3 sputum samples are optimal 93% 100% 100% 81% 50% 0% First Second Third
- 13. These data have helped form the basis of the current three-specimen requirement for ruling out pulmonary TB. The probable rationale behind this recommendation is that some patients shed mycobacteria irregularly and in small numbers and, thus, increasing the number of specimens would increase the yield. With regard to the recommendation for early morning specimens, the assumption is that M. tuberculosis would be present in maximum concentration in sputum after pooling overnight in the respiratory tract.
- 14. Specimen collection timing • Spot-Morning-Spot WHO/IUATLD recommendation • Spot - 1st visit to the clinic • Early morning- first sputum in the morning • Spot - 2nd visit to the clinic
- 15. Instructions to Patients Give clear instructions to patients on how to collect the sputum: Importance of sputum examination for TB diagnosis Need for real sputum, not saliva How to produce good sputum How to open and close the container How to avoid contamination of outside
- 16. Patient education: the best specimen comes from the lungs
- 17. • Sputum should be collected under direct observation, at least for the first time. This is to insure that the patient is being properly coached and is giving a good coughing effort, as well as insuring that uncooperative patients are producing their own sputum for examination.
- 18. INSTRUCTION FOR SPUTUM COLLECTION 1. Specimen collection should be done without the client rinsing the mouth or brushing teeth 2. If possible, go outside or open a window before collecting the sputum sample. This helps protect other people from TB germs when they cough. 3. The cup is very clean. Don’t open it until you are ready to use it. 4. Inhale 2-3 times, breathe out hard each time 5. Cough deeply from the chest 6. Place the container close to the mouth to collect the specimen
- 19. CON’T 7. Spit the sputum into the plastic cup. Avoid contaminating the inside of the container and lid 8. Keep doing this until the sputum reaches the 5 ml line (or more) on the plastic cup. This is about 1 teaspoon of sputum. 9. Screw the cap on tightly so it doesn’t leak. 10. Write on the specimen bottle the patient’s name, hosp number, the number of sputum collected, the date of collection 11. Put the cup into the specimen bag and into a specimen box 12. Submit this to the laboratory ASAP
- 20. • If the patient cannot cough up sputum, advice him/her to try breathing steam from a hot shower or a pan of boiling water.
- 22. Packing/Transportation Specimens Tightly cap labeled sputum bottle Cushion a box or carton with cellulose(optional) Pack 3 specimens (well labeled) of same patients in 1 biohazard specimen bag Place bag of specimen upright in the prepared box
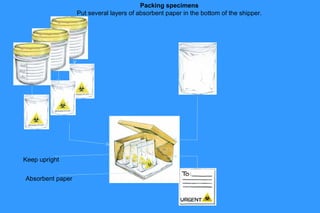
- 23. Packing specimens Put several layers of absorbent paper in the bottom of the shipper. Keep upright Absorbent paper
- 24. Packing/Transportation Specimens • Place the request form in the pocket of the plastic • Prepare a checklist and place it in a separate bag • Seal and label the box of specimen • Send to nearest Health facility/hospital or laboratory.
- 25. Sputum sample What is a good sample? • 3-5 ml • Usually thick and mucous, but may be fluid with pieces of purulent material • Color varies from opaque white to green, reddish to brown when blood is present • Clear saliva is not suitable; but examine saliva if a better specimen can not be produced, especially for follow-up examinations • A specimen mainly containing blood should not be examined; patient should see a doctor for immediate management
- 26. Best use of Collection Container • A new container per patient • To obtain a good quality sample, several attempts by patient may be necessary. • Use a new container on each attempt • Patient to open container only when about to collect specimen • Container once opened should be considered used (dispose accordingly)
- 27. Best container?? • • • • • • Clean Plastic Transparent Wide mouth Good seal Easily labelled
- 28. Good Quality Sputum Good quality sputum( mucoid) Good quality sputum(purulent) Blood stained
- 29. Poor sputum quality saliva or nasal secretions are unsatisfactory. Saliva from mouth is water and thin. Good sputum quality Sputum from lungs is usually thick and sticky
- 30. Where can you store the sputum? • You can store the cup in the refrigerator overnight if necessary. Do not put it in the freezer or leave it at room temperature.
- 31. Diagnosis
- 32. In Fiji, TB is diagnosed using the following Lab methods 1) smear microscopy – to look for the bacteria (AFB) microscopically 2) Sputum culture - to grow the bacteria Mycobacterium tuberculosis 3) GeneXpert MTB/RIF - cartridgebased, automated that can identify Mycobacterium tuberculosis(MTB) and resistance to Rifampicin(RIF) within 2hr period
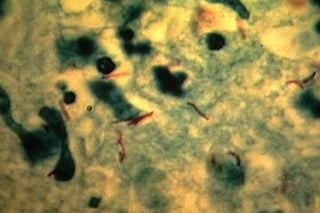
- 33. 1. Direct Microscopy M. tuberculosis is a acid–fast bacterium, rod-shaped bacterium measuring 2-4 x 0.2-0.5 μm. They appear as bright red rods against a contrasting background. The Ziehl-Neelsen stain is used to demonstrate the presence of the bacilli in a smear. The technique is simple, inexpensive and detects those cases of tuberculosis who are infectious. M. tuberculosis appearing as bright red bacilli (rods) in a sputum smear stained with the Ziehl-Neelsen stain
- 34. AFB MICROSCOPY Advantages -Rapid - High specificity (AFB in sputum = TB) • All mycobacterium are acid fast, no exception ; • > 98% for AFB in high burden countries - Accurate diagnoses - Using simple and available equipment Disadvantage Low sensitivity; culture Reported sensitivity ranging 25 to 65% when compared to Species differentiation impossible. False positive; mycobacteria. Saprophytic
- 35. Reporting on AFB Microscopy Number of bacilli seen Result reported None per 100 oil immersion fields Negative 1-9 per 100 oil immersion fields Scanty, report exact number 10-99 per 100 oil immersion fields 1+ 1-10 per oil immersion field 2+ > 10 per oil immersion field 3+
- 36. 3 smears = sensitivity of 1 culture About 95% of infectious cases
- 40. Laboratory Diagnosis 1- Sputum smears stained by Z-N stain Three morning successive mucopurulent sputum samples are needed to diagnoise pulmonary TB. Advantage: - cheap – rapid - Easy to perform - High predictive value > 90% - Specificity of 98% Disadvantages: - sputum ( need to contain 5000-10000 AFB/ ml.) - Young children, elderly & HIV infected persons may not produce cavities & sputum containing AFB.
- 41. Importance of AFB microscopy • Sputum smear microscopy - the most efficient tools of case finding in a national tuberculosis control program because of its ability to identify and distinguish the cases with highest priority in tuberculosis control. • It is dependable if sputum specimens are of GOOD QUALITY.
- 42. AFB smear-microscopy Acid-fast bacilli (AFB) (shown in red) are tubercle bacilli
- 43. Acid fast smear showing TB bacilli
- 44. 2. TB cultures
- 45. demiology 3 Culture M. tuberculosis grows in Lowenstein Jensen medium or Ogawa medium, which contains inhibitors to keep contaminants from outgrowing the organism. Because of its slow growth, it takes 6-8 weeks before small buff-coloured colonies are visible on the medium. Typical small, buff coloured colonies of M. tuberculosis on Lowenstein Jensen medium
- 46. Identification from solid media • Rate of growth: visible isolated colonies in 2–4 weeks. • Colony morphology: – buff-coloured (never pigmented) – rough – waxy – appearance of bread crumbs or cauliflower. From colonies , ZN staining should be performed 46
- 48. 3. GeneXpert MTB/RIF Test
- 49. TEST CRITERIA Xpert MTB/RIF test should be done on; 1.Symptomatic patients with AFB negative (negative on 3 specimens) 2.Relapses suspect cases 3.HIV patients 4.Cases from high burden (MDR) countries OTHER REQUIREMENTS •Request forms for TB testing should be used •Clinicians should complete request form with clinical diagnosis •Specimen collected should be of good quality •Lab Technicians should only do GeneXpert test if diagnosis falls on any of the criteria above. 1.AFB positive not converting at 3 months of treatment
- 50. How could GeneXpert help? • Low sputum smear positivity of PTB in HIV patient is common due to: – Poor immunity to localize the infection – High possibility for TB dissemination to other organs GeneXpert can detect more TB among PLHIV
- 51. GeneXpert - Facts and Background • • Endorsed by WHO : 8 DEC 2010 18 months of rigorous assessment of its field effectiveness in: Early diagnosis of TB, as well as MDRTB TB complicated by HIV infection, which are more difficult to diagnose • The test could revolutionize TB care!
- 52. How does the test work? • Detects DNA sequences specific for Mycobacterium Tuberculosis and Rifampicin resistance by PCR • Based on Nucleic Acid Amplification Test (NAAT). The Xpert® MTB/RIF – – – – purifies concentrates amplifies (by real-time PCR) and identifies targeted nucleic acid sequences in the Mycobacterium tuberculosis genome,
- 53. TB point of care testing • Simple • Minimum 3 steps for sample preparation • Rapid • 2 hours result availability • Accurate • Sensitivity : adult PTB Smear+Culture+ = 95% • Smear- Culture+ = 80% • Specificity : adult PTB = 95%
- 54. GeneXpert test is available in Fiji • 3 GeneXpert diagnosis sites in the microscopic centres of National TB Programm supported by WHO/ Global Fund Grant, Fiji: Labasa Hospital, Lautoka and PJ Twomey Hospital. • Testing done for: Sputum Smear suspects case MDR suspect cases and PLHIV
- 55. Take-home messages • Sputum smear microscopy - the most efficient tools for detecting TB with highest priority in TB control hence is dependable if sputum specimens are of GOOD QUALITY. • Sputum collection procedures needs to be stregthened
- 56. THANK YOU •Any burning questions????
- 57. SPUTUM SALIVARY DATA, 1st Qtr HEALTH FACILITY TOTAL SPECIMENS POOR SAMPLE QUALITY DIAGNOSTIC / FOLLOW UP DIAGNOSTIC / FOLLOW UP LABASA 155 58 SAVUSAVU 46 22 LEKUTU 2 0 WAINUNU 2 1 SEAQAQA 6 3 2 2 3 2 NABOUWALU 8 5 WAINIKORO 8 4 TAVEUNI 39 23 UNSPECIFIED 1 0 TOTAL 272 120 NAVAKAKA N/STATION DREKETI % salivary 48
- 58. SPUTUM SALIVARY DATA, 2nd Qtr HEALTH FACILITY TOTAL SPECIMENS POOR SAMPLE QUALITY DIAGNOS TIC FOLLOW UP DIAGNOSTI C FOLLOW UP LABASA 113 21 38 8 SAVUSAVU 39 2 12 0 LEKUTU 1 0 0 0 NADURI3 3 0 3 0 SEAQAQA 6 0 1 0 DREKETI 2 0 0 0 RABI 8 1 2 1 WAINIKORO 10 0 6 0 TAVEUNI 5 1 2 0 TOTAL 187 25 64 9 % Salivary 31