Lab dia of parasite
- 1. LABORATORY DIAGNOSIS OF PARASITic infection Prabin Shah BScMLT, MSc(Biochemistry)
- 2. Specimens • Faeces • Blood • Bone marrow • Urine • Biopsy material from spleen, liver, lymph nodes.
- 3. Collection of the specimen • Collect in wide mouth clean container without contamination of urine, water or disinfectants. • Collection of three faecal specimen to make the diagnosis of intestinal parasitic diseases. • Two specimen obtained successive days during normal bowel movement • 3rd is after magnesium purge. • Liquid specimen should be examined within 30 min • Semi formed stools within 60 min after collection for suspected infection like E. histolytica, Giardia lamblia. • Blood sample: finger prick, lobe of an ear or anti coagulated blood can be used
- 4. Preservatives • Formalin solution. • Polyvinyl alcohol • Merthiolate – iodine – formalin solution (MIF) • Schaudinn’s solution.
- 5. Macroscopic examination of faeces • Consistency • Color • Odor • Presence of blood or mucus • If the presence of blood and mucus in stool (suggestive of amoebic dysentery)
- 6. Microscopic examination • Saline wet mount • Iodine wet mount • Smear after concentration • Stained smear
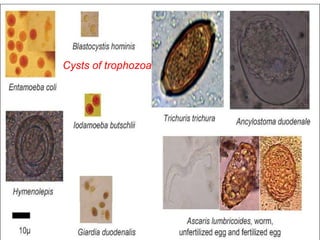
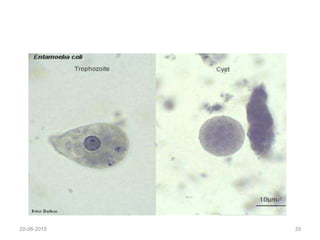
- 7. Saline wet mount • Small quantity of faeces is diluted with normal saline (0.9%) placed on clean glass slide, and cover with cover slip. • Smear is examined under microscope. • It is used to detect tropozoites and cysts of tropozoa and eggs and larvae helminthes. • It detect live motility of E. histolytica, Giardia lamblia and Balantidium coli.
- 8. 20-06-2015 8 Cysts of trophozoa
- 11. Iodine wet mount • Stool is emulsified in a drop of five times diluted solution of Lugol’s iodine on a clean glass slide. • Covered with cover slip and examined under microscope. • It is used to identify the nuclear character of cysts and tropozoites. • Lugol’s iodine; • 10 gm potassium iodide • 100ml D/W • 5gm iodine crystal
- 12. Wet preparation • A drop of anti coagulated blood can be placed on a clean glass slide. • Cover the cover slip and examined under microscopically for large, motile, exo-erythrocytic parasites. such as : trypanosomes and microfilariae.
- 13. Microfilaria
- 14. Concentration methods Floatation technique • Saturated salt floatation technique • Zinc sulphate centrifugal floatation technique • Sedimentation technique • Simple sedimentation • Formalin ether sedimentation 20-06-2015 14
- 15. Saturated salt floatation technique • Take 1 gm of faeces is placed in a flat bottomed vial and add 5 drop of saturated salt solution are added. • stirred with a glass rod to make an emulsion. • More salt solution is added so that the container is nearly full, mix solution. • A glass slide is carefully laid on the top of the container and stand for 20 – 30 min. • After which the glass slide is quickly lifted, turned over smoothly and examine under microscope 20-06-2015 15
- 16. • It has been observed all the eggs except unfertilized of A. lumbricoides, eggs of Taenia solium, T. saginata float in saturated salt solution. 20-06-2015 16
- 17. Zinc sulphate centrifugal floatation technique • About 1 gm of faeces is thoroughly mixed in 10 ml of lukewarm distilled water. • The filtrate is poured in to a 15 ml conical centrifugal tube and centrifuged at 2,500 RPM for 1 min. • The clear supernatant is poured off and 3 – 4 ml of zinc sulphate is added to sediment up to the top of tube. • Again centrifuge at 2,500 RPM for 1 min. 20-06-2015 17
- 18. • With the platinum wire loop sample is taken from the surface on to a glass slide and put cover slip observe under microscope. • For protozoal cysts, one drop of iodine solution is added before the cover slip is put on. • This technique effectively concentrates cysts of protozoa, eggs of nematodes, and small tapeworms. 20-06-2015 18
- 19. Eggs of nematodes 20-06-2015 19
- 20. Simple sedimentation • A sufficient amount of faeces is thoroughly mixed with 10 – 20 time its volume of tap water. • Allow to settle in a cone – shaped flask for an hour or two hrs. • This process is repeat till supernatant fluid is clear. • Take the sediment at the bottom examined under microscope for the eggs. 20-06-2015 20
- 21. Formalin – ether sedimentation • Half teaspoon if faeces is thoroughly mixed in 10 ml of water and strained through two layers of gauze of funnel. • The filtrate is centrifuged at 2,000 RPM for 2 min • The supernatant is discarded and the sediment is resuspended in 10 ml of saline. • Again centrifuge and discard the supernatant and add 7 ml of formalin saline and stand for 10 min. • To this is add 3ml of ether the tube is stopperde and shaken vigorously to mix and centrifuge for 2 min. 20-06-2015 21
- 22. • The four layers are visible, the top layer is consist of ether, 2nd plug of debris, 3rd is clear layer formalin saline and 4th is sediment. • Take a sediment on clean glass slide and examine under microscope. 20-06-2015 22
- 23. Quantification of worm burden • There are two methods; • Direct smear egg count • Stoll’s method • Direct smear egg count • Two mg of faeces is mixed in a small drop of saline on a slide and cover the cover slip. • Examined under low power of the microscope and count the eggs and calculate the number of eggs / gm of faeces. 20-06-2015 23
- 24. Stoll’s method • Commonly used method for determination of helminthic eggs in faeces. • 4gm of faeces is mixed with 56 ml of N/10 NaOH in a flask to make a uniform suspension. • This is facilitated by adding the glass beads and closing the mouth with a rubber and shake vigorously. • 0.075 ml of emulsion is removed with pipette and placed on a glass slide and put cover slip. • Count the eggs in low power of the microscope • Calculate the egg/gm of faeces multiplied by 200. 20-06-2015 24
- 25. Anal scraping and swabs • Amoebiasis cutis of the perianal area may be diagnosed by demonstrating motile tropozoites of E histolytica in material scraped from ulcer and examined in saline suspension on a slide under cover slip. • E. vermicularis infection is usually diagnosed by demonstrating the presence of egg on the perianal and perineal skin. Following methods are; • Scotch cellulose adhesive tape method • NIH swab. 20-06-2015 25
- 26. Scotch cellulose adhesive tape method • A 3 inch of the tape is held adhesive – side – out on the end of a wooden tongue blade by the thumb and index finger. • The adhesive surface of the tape is then pressed against the perineal skin at several places. • Then placed the adhesive – side – down on the slide for examination. • A drop of toluene may be placed between the tape and slide. • The toluene clear essentially everything except eggs and hair. 20-06-2015 26
- 27. NIH swab • Eggs are deposited in large number on the perianal and perineal skin at night can be demonstrated by scraping this area by NIH swab in the morning before taking bath. • Spread over glass slide and examined microscopically. • This procedure should be repeated on three successive days. 20-06-2015 27
- 28. Examination of urine • The specimen is collected in a sedimentation of glass, and the eggs are allowed to sink to the bottom. • A drop of sediment is placed on the glass slide and examine under microscope for Trichomonas Vaginalis. 20-06-2015 28
- 30. Examination of sputum • The sputum is spread in a Petri dish and material suspected of bearing eggs or pus and Charcot – Leyden crystals is placed on a glass slide. • Cover the cover slip and examined under microscope for Paragonimus westemani. 20-06-2015 30
- 31. 20-06-2015 31
- 32. Permanent stained smear • Iron – haematoxylin stain • Trichrome stain • Modified acid – fast stain. 20-06-2015 32
- 33. Iron – haematoxylin stain • A thin smear of faeces is made on a clean, glass slide. • Keep the slide in Schaudinn’s solution for 15 min for fixation of the smear. • The smear is immersed in 70% and 50% alcohol, 2 – 5 min in each. • Wash in tap water for 2 – 10 min, immersed in 2% aqueous ferric ammonium sulphate solution for 5 – 15 min. • Wash in running tap water for 3 – 5 min. 20-06-2015 33
- 34. • Then stained in 0.5% aqueous haematoxylin for 5 – 15 min and wash in running tap water for 2 – 5 min. • Then differentiated in saturated aqueous solution of picric acid for 10 – 15 min. • Dehydrated by immersion for 2 – 5 min each in 50%, 70%, 80%and 95% alcohol and 5 min each in two changes of absolute alcohol. • Stained smear is then cleared in two changes of xylol for 3 – 5 min each, mount in Canada balsam and cover the cover slip. 20-06-2015 34
- 35. 20-06-2015 35
- 36. Trichrome stain • Fixation is same as Iron – haematoxylin stain. • Then it is stained with Trichrome solution for 10 min and differentiated in acid alcohol for 2 – 3 seconds. • Rinse in absolute alcohol in several times and dehydrated in two changes of absolute alcohol for 2 – 5 min each. • Stained smear is then cleared in two changes of xylol for 2 – 5 min each, mount in Canada balsam and cover with cover slip. 20-06-2015 36
- 37. Modified acid – fast stain • It is detection and identification of Cryptosporidium parvam and Isospora belli. • Thin smear of faeces is made on a clean glass slide and fix by heat at 70⁰C for 10 min. • keep the slide on staining rack and add carbol fuchsine and heat the stain till stars steaming allow for 9 min. • Wash it in tap water and decolorized with 5% sulphuric acid for 30 seconds. • Wash the slide and add counter stain with methylene blue for 1 min. • Wash it in tap water, dried, mount in Canada balsam and cover the cover slip. • The acid fast Cryptosporidium oocyst stain red with carbol fuchsin and non acid fast background is blue. 20-06-2015 37
- 38. 20-06-2015 38
- 40. EXAMINATION OF BLOOD FOR PARASITES • Examination of thin blood film: • Preparation: • Prick the finger or ear lobe with surgical cutting needle under aseptic condition. • Take a drop of blood not larger than a pin’s head on a clean glass slide. • Make smear with the help of spreader. • Dry the smear and labeled the smear. 20-06-2015 40
- 41. STAINS: with Leishman’s stain • Pour the stain on smear and allow it for 2 min . Dilute the stain with twice its volume of D/W which should be neutral. o Allow the diluted stain to remain on the slide for 10 – 15 min. o Wash the slide under running tap water, and dry the smear. o The stained smear is observed under oil – immersion lens. 20-06-2015 41
- 42. 20-06-2015 42 p. vivax P. palcifarum
- 43. EXAMINATION OF THICK BLOOD FILM Preparation : o A big drop of blood is taken on a glass slide and spread with a needle or corner of another slide. o The thickness of the film should be such as to allow a newsprint to be read through the preparation. o Dry the smear in horizontal position and kept by cover by Petri dish. o Drying may be accelerated by putting the slide inside an incubator. 20-06-2015 43
- 44. STAINING : o By Leishman’s or Field’ stain, DEHAEMOGLOBINISATION: o The film is flooded with the mixture of glacial acetic acid and tartaric acid. o Then fixed with methyl alcohol for 3 – 5 min and wash in D/W. o After dehaemoglobinisation stain the smear in Leishman’s stain same as the thin smear. 20-06-2015 44
- 45. 20-06-2015 45 P. vivax P. palcifarum
- 46. EXAMINATION OF BLOOD FOR MICROFILARIAE o A thick film of blood is prepared and kept covered. Next morning it is, dehaemoglobinised by putting the slide in water. o Then dry and fixed in methyl alcohol, then stain with Leishman’s stain. o Occasionally the microfilaria may be detected even in thin smear. MICROFILARIA COUNT : The total number of microfilariae in the thick smear multiplied by 50 will give the no per ml of blood. 20-06-2015 46
- 47. Microfilaria
- 48. J. S. B. Staining For thin smear: • Fix the smear in methyl alcohol for 3 – 5 min and allow to dry. • Immersed in solution I for 30 sec wash in tap water. • Stain with solution II for 1 sec wash again in tap water for 4 sec. • immersed in solution I again for 30 sec, wash in tap water for 10 sec, dried and examine under microscope. 20-06-2015 48
- 49. Blood concentration methods • Microhaematocrite centrifugation. • Triple centrifugation. • Buffy coat concentration. • Knott concentration. • Membrane filtration. • Gradient centrifugation. 20-06-2015 49
- 50. Culture NNN medium • NNN medium for the diagnosis of leishmaniasis. • It consist two part of salt agar and one part of defibrinated rabbit blood. • Blood, aspirates or small biopsy sample from spleen, liver or bone marrow inoculated in to water of condensation of the medium and incubated at 22 – 25⁰C. • the amastigote form change in to promastigote form which multiplied rapidly to produce a large number of flagellates in bottom of the tube. 20-06-2015 50
- 51. 20-06-2015 51
- 52. Hockmeyer’s medium • This medium consists of Schneider’s commercially prepared insect cell culture medium. • In addition of 30% heat inactivated foetal calf serum and 100 IU penicillin and 100 micro g streptomycin /ml. • The medium is inoculated with specimen incubated at 22 – 25⁰C and examine the promastigote by microscopically. • G. lambiancan be grow axenically in Diamonds medium, used for axenic cultivation of E. histolytica. • 20-06-2015 52
- 53. Serologic diagnosis test Disease Indirect haemagglutination test (IHA) Amoebiasis, cystcercosis, echinococcosis, filariasis, stronglyoidiasis, fascioliasis, changas’ disease. Fluorescent antibody test (FAT) African trypanosomiasis Indirect fluorescent antibody test (IFAT) Leishmaniasis, malaria, schistosomiasis, toxoplasmosis. Enzyme linked immunosorbent assay (ELISA) Toxoplasmosis, toxocariasis, ascariasis. Compliment fixation test (CFT) Chagas’ disease, paragonimiasis, leishmaniasis. Latex agglutination test Echinococcosis Bentonite floculation test Trichinellosis, echinococcosis.20-06-2015 53
- 54. Molecular assays • DNA probe; used for diagnosis of malaria and filariasis. • Polymerase chain reaction (PCR); used for Leishmaniasis, toxoplasmosis, Chagas’ disease, onchocerciasis. 20-06-2015 54
- 55. References • Medical parasitology - D.R. ARORA, B. ARORA 2nd edit - K. D. CHATTERJEE 13TH edit 20-06-2015 55
- 56. 20-06-2015 56