laboratory diagnosis of fungal inections
- 1. Laboratory Diagnosis Of Fungal Infections Dr Aditi Kothari
- 2. Overview • Classification • Specimen collection • Transport of specimen • Processing of specimen 1. Direct examination 2. Fungal culture 3. Non culture based methods 4. Immunologic and Serologic tests for fungal infections 5. Molecular methods 6. Recent techniques • Reference
- 5. Classification based on clinical manifestation • Superficial mycosis • Subcutaneous mycosis • Systemic mycosis • Opportunistic mycosis
- 6. Specimen collection General considerations • Always clean the site with 70% isopropyl alcohol first before collection (specimens often contain other bacteria or fungi that rapidly overgrow). • Proper collection of specimens and rapid transport is crucial to recover fungi. • Collect sufficient amount of sample. • Use sealed sterile containers for liquid or moist specimen . • Skin scrapings, flakes, nail fragments, and hairs can be transported dry in an sterile autoclaved envelope. • Specimens that are not processed immediately should be held at room temperature (viability of fungi decreases over time).
- 7. Specimen collection • Specimens can be refrigerated for a short time, except for dermatologic specimens (skin, hair, nails), blood, and cerebrospinal fluid (CSF). • Csf sample for Cryptococcus neoformans, sputum for Histoplasma capsulatum, and skin scraping for Blastomyces dermatitidis do not survive well in frozen or iced specimens so should not to be refrigerated )
- 8. Sample rejection criteria • Absence of patient identification on the container • A dried-out swab is received or the material collected is insufficient in volume • The sample is submitted in an improper container or in an unsuitable condition
- 9. Type of specimen Recommended procedure Transport Sputum 1ST early-morning sample after vigorously rinsing mouth with water immediately before coughing 1/2–1 oz of sputum into a sterile, screw-capped container. Induced sputum in case of children Bronchoscopy Transported promptly in sterile, sealed containers Cerebrospinal fluid As much as possible Aprox-5ml Do not freeze Urine Early-morning urine sample is preferred; random samples are acceptable. Specimens collected in sterile, screw-cap containers and sent immediately. A delay in processing beyond 2 hours , the sample should be refrigerated at 4°C to inhibit overgrowth of bacteria. Specimen collection and transport
- 10. Specimen collection and transport Type of specimen Recommended procedure Transport Prostatic secretions Deep-seated mycoses eg: Blastomycosis, Histoplasmosis Or Coccidioidomycosis Exudates Aspiration of exudates using a sterile precautions and sent to lab in sterile container Blood A fungal blood culture that utilizes a lysis-centrifugation systems i.e. Isolator (Wampole Laboratories, Cranbury, NJ)for the recovery of H. capsulatum. As well as standard blood culture Sterile blood culture bottles
- 11. Specimen collection and transport Type of specimen Recommended procedure Transport Skin Sample is taken from the peripheral, erythematous, growing margin of typical “ringworm” lesions by scraping with the edge of a scalpel blade Sample should be labelled and packed in a sterile autoclaved brown /black paper Collect as much as sample from affected area for better yield of organism Hair Hair should not be cut but should be plucked with forceps from the affected area or those that fluoresce under the woods lamp Hair follicle as well as ¾ of hair from the hair blub should be collected Nail Nails should be cut with a nail clipper And debris beneath the nail should be collected in a sterile brown paper The proximal part of the affected nail can be scraped off Corneal scraping Scraping from base and margins using kimura’s spatula Aspirate can be taken from hypopyon or endophthalmitis KIMURA’S SPATULA
- 12. fungal infection of nails Fungal infection of hair in a 1yr old (tinea captis)
- 13. Wood lamp examination Fluorescence in tinea capitis using Wood lamp
- 14. Fungal infection of skin (tinea corporis)
- 15. Staining techniques in mycology Wet preparations • Potassium hydroxide mount • India ink stain • Nigrosin stain • Calcofluor white stain • Lactophenol cotton blue • Neutral red stain Staining methods • Grams stain • H &E stain • Giemsa • PAS • Gomori’s methamine stain • Acridine orange stain • Flourescent antibody stain
- 16. Potassium Hydroxide Mount (KOH) • Primary screening tool • Soften and digest protein debris and dissolves keratinized cells so that fungal elements can be clearly visualized • Warming and overheating • Concentration of KOH may range from 10 - 40% • Overnight incubation is required for specimens like nail clippings skin biopsy 10 %KOH - 10gms 10%Glycerol – 10ml (prevent drying) Distilled water – 80ml Koh mount showing septate hypha and mycelium
- 17. Modification of KOH Dimethyl sulfoxide(DMSO) is added to 20% KOH Useful cleansing agent , Heating of slide is not required (20gms KOH,4Oml DMSO, 60ml Distal water (DW)) 20% KOH and equal volume of parker ink – Malassezia spp 20% KOH and a drop of acridine orange – fluorescent microscopy KOH with calcofluor white – increasing sensitivity test • Examine under 40X in a ploughing manner • To see yeast cells ,hyphae, pseudo hyphae
- 18. India Ink Stain/Prepration (IIP) • Negative staining • For encapsulated organisms • Modification – 2% chromium mercury Help to visualize external capsule • Dry India ink preparation – mounting India ink with DPX solution to preserve slides India ink -150ml Merthiolate (1:1000) 3ml (thimerosol- protect contamination) Tween80 (1:10,000)- 0.1ml (help reduce bubble formation) Cryptococcus seen on IIP
- 19. Nigrosin stain • Negative staining • Better than India ink - Has more shelf life - No carbon particles appear in stain solution - Has antibacterial property so contamination is comparatively less Contents Nigrosin granules 10gms Formalin (10%)- 100ml Negative staining –by nigrosin stain
- 20. Calcofluor –white stain (CFW) • Fluorescent dye • Specifically stains chitin in the cell wall • Can be coupled with KOH- for better sensitivity • Principle- • Binds specifically to beta linked polysaccharides in the cell wall which is visualized when exposed to a longer wavelength of light • Light blue in colour when seen in fluorescent microscope CFW-showing yeast cells and hyphae
- 21. Modification of CFW Blankophor(1%) Less artifacts Does not get precipitated while mixing with KOH Specifically used for Pneumocystis And Microsporidium spp
- 22. Lactophenol cotton blue (LPCB) • To study the morphological features of the fungus • Stains the outer wall of the fungus • LPCB is performed after colonies appear in culture media Contents Melted phenol-20ml (disinfectant) Lactic acid – 20ml (preserves fungal morphology) Glycerol-40ml (prevents drying) Cotton blue- 0.05gms (stains outer wall of fungus) Distilled water- 20ml LPCB of Penicillium spp
- 23. Modifications of LPCB LCB with polyvinyl alcohol -Can be permanently preserved PHOL STAIN –formalin is used instead of phenol and methylene blue instead of cotton blue Narayan stain-which contain DMSO, methylene blue and glycerine
- 24. Neutral red stain • Used to check viability of fungus • Principle- stain is capable of passing through intact plasma membrane and is stored in lysosome of viable cell Used for dermatophytes and Candida spp
- 25. Diazonium Blue B Stain (DBB) • Used for differentiation of basidiomycetous spp –Trichosporon and ascomyceteous Geotrichum spp • Dark red colour for Trichosporon within 2 min of staining • Where as Geotrichum are DBB negative i.e. they remain unstained Also used for identification of yeast
- 26. Differential stains Stain Observation Components it stains Remarks Gram’s stain Gram positive Violet coloured Oval budding yeast cells ,hyphae & pseudo hyphae Modified Acid-Fast stain Pink coloured filamentous bacteria Actinomycetes Nocarida spp Giemsa stain Nuclei- purple Cytoplasm-blue RBC-pink Intracellular yeast cells H.capsulatum PAS Fungi- magenta red Nuclei- blue For fungi in tissue Living fungus H &E stain Fungi-blue RBC-red Gomori’s methamine stain (GMS) Fungai- brown to black Mps-grey Tissue -pale green For polysaccharide contents of fungus in tissue Pneumocystis spp Both dead and live fungus And filamentous bacteria Stain Observation Components it stains Remarks Gram’s stain Gram positive Violet coloured Oval budding yeast cells ,hyphae & pseudo hyphae Modified Acid-Fast stain Pink coloured filamentous bacteria Actinomycetes Nocarida spp Giemsa stain Nuclei- purple Cytoplasm-blue RBC-pink Intracellular yeast cells H.capsulatum PAS Fungi- magenta red Nuclei- blue For fungi in tissue Living fungus H &E stain Fungi-blue RBC-red Gomori’s methamine stain (GMS) Fungai- brown to black Mps-grey Tissue -pale green For polysaccharide contents of fungus in tissue Pneumocystis spp Both dead and live fungus And filamentous bacteria
- 27. Other staining technique Stain Observation Components it stains Remarks Acridine orange stain RNA - red Fungus –bright orange DNA –green Stains nucleic acid Florescent antibody stain Antibody coated fungi can be demonstrated Detects fungal ag in smear and sections Organisms is scanty Masson Fontana stain (MSF) Melanin granules reduces silver present in the stain Hyphae- black (only phaeoid fungus) Nucelli-red Dematiaceous fungus Irregular septations and beaded forms GMS stains all elements black with green background Melanin based staining Mayer mucicarmine stain Polysaccharide stain Cryptococcus –deep rose red Nuclei –black Tissue –yellow Cryptococcus and Rhinosporidium spp Schmorl’s melanin stain Uses reducing properties of melanin Fungai- bluish green Nuclei-pinkish red Cytoplasm- pale pink Melanin based staining Better than MFS
- 28. Culture media • Basal media • Nutritional deficient media • Enriched and selective media • Differential agar media • Media for stimulation of ascospores • Media used for biochemical test Colonies of Cryptococcus neoformans growing on niger seed (i.e., birdseed) agar. The darkly pigmented colonies are characteristic of C. neoformans
- 29. Basal media Media Remarks Sabouraud dextrose agar(SDA) Peptone-10gm (growth) 4%Dextrose- 40gms (carbon source) Agar-20gms DW-1000 ph-5.6 Test tube- adequate surface area Min aerosol production Slow drying Neutral SDA Ph-6.8-7 2%Dextrose 20gms(increases rate of isolation) neopeptone For isolation of opportunistic and dimorphic fungi Preferred for Blastomyces dermatitidis SDA with antibiotics SDA Cyclohexamide--500mg Chloramphenicol-- 50mgs Gentamicin---20mg Avoid bacterial and saprophytic fungal contamination 2nd slant without cycloheximide should be inoculated (SDA with this antibiotics inhibit most of the moulds and yeast) Candida glabrata on Sabouraud’s dextrose agar after 3 days of incubation
- 30. Nutritional deficient media Media Remarks Corn meal agar Corn meal- 8gms Agar-4gms DW-200ml Tween 80 2ml (decreases surface tension) For stimulation of clamydospore formation (Dalmau plate culture) Rice starch agar Cream of rice-2gms Tween80-2ml Agar-4gms DW-200ml Faster formation of Chlamydospores in Candida spp Cornmeal preparation of C. albicans demonstrates chlamydospores (arrow) and regular clustering of blastoconidia.
- 31. Enriched And Selective Media Media Uses Brain heart infusion agar With antibiotics (M TO Y Y TO M ) Histoplasma capsulatum Blastomyces dermatitidis primary isolation as well as invitro conversion Cysteine heart and haemoglobin agar Similar to BHIA Biphasic medium To isolate organism from blood Bird seed agar(creatinine) /Niger seed agar Cryptococcus spp black colour colonies Caffeic acid is oxidized by phenoloxidase into melanin Blood agar Histoplasma capsulatum Blastomyces dermatitidis
- 32. Enriched And Selective Media Media Uses Dermatophytes test medium Incubated at 25ᵒC Change in colour in 3-6 days (phenol red) Identification of dermatophyte Imparts red colour if dermatophytes are present Dermatophyte identification medium Incubated at 37ᵒC Colour changes from blue to purple Presence of dermatophytes indicated by change in colour of medium by greenish blue to purple within 48hrs Primary isolation of dimorphic fungi Malt extract agar In place of SDA (early growth and sporulation is better) Maintainance of stock culture Potato dextrose agar Stock culture Sporulation Differentiation of dermatophytes on the basis of pigment production Leeming and Notman agar And modified Dixon agar (favoured) Malassezia spp
- 33. Differential agar Media Remarks CHROM agar 30ᵒC for 48-72 hrs Presumptive identification Direct detection of enzymatic activity Fluorochromes are added Multiple species of candida can be detected Candida albicans – cream to light pink Candida parapsilosis – red to maroon Candida tropicalis –red to maroon Candida krusei – white to cream spreading type of colonies.
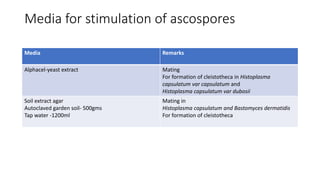
- 34. Media for stimulation of ascospores Media Remarks Alphacel-yeast extract Mating For formation of cleistotheca in Histoplasma capsulatum var capsulatum and Histoplasma capsulatum var dubosii Soil extract agar Autoclaved garden soil- 500gms Tap water -1200ml Mating in Histoplasma capsulatum and Bastomyces dermatidis For formation of cleistotheca
- 35. Media used for biochemical test Medium Used for Tetrazolium reduction medium Peptone 1gm Glucose 4gm Beef extract 0.1 gm Tetrazolium 20mg Neomycin 50mg Distilled water 100ml Adjust pH 5.6-6.2 Differentiate Candida spp CANDIDA SPECIES Candida albicans- pale pink colour C .tropicalis - orange pink C . pseudotropicalis -salmon pink C .parapsilosis -rose pink C .gulliermondii - Pink and pasty C . krusei -pink and dry C . glabrata- pale pink Urease test Positive –pinkish red T mentagrophyte –deep red T rubrum – negative Cryptococcus – rapid urease test positive Trichosporon- positive Rapid urease test Results within minutes
- 36. Media Remarks Carbohydrate fermentation test 2% sugar Peptone 1% NaCl 0.5% Andrade’s indicator 0.005% Sugars Production if gas bubbles in Durham’s tube indicates presence of gas And change of colour of media indicates fermentation of sugar Carbohydrate assimilation test Inability to assimilate certain sugar is because substrate is not able to enter the yeast cell Or absence or enzyme
- 37. Urease test
- 40. Non culture based methods • Vinyl tape or scotch tape method A. The sticky side of tape being pressed to the surface of the fungus colony. B. Transparency tape preparation: stretching the inoculated tape over a drop of lactophenol aniline blue on the surface of a microscope slide.
- 41. LPCB Tease mount preparation- dissection of the colony fragment with needles in a drop of lactophenol aniline blue prior to placement of the coverslip.
- 43. Germ tube test • Also known as Reynolds-Braude phenomena • Used for identification of Candida albicans and Candida dubliniensis • Light suspension of Colony of candida spp is emulsified with 0.5ml of mammalian serum And incubated at 37ᵒC for 2hrs GT are seen as long tube like extension seen from yeast cell with no constriction at the point of attachment Longer incubation beyond 4hrs – other hyphae producing yeast might begin to germinate beyond this time New milk medium and amino acid synthetic medium can also be used
- 44. demonstration of germ tube
- 45. Hair perforation test • To differentiate between T. mentagrophytes and T. ruburm • As well as M. canis and M. equinum • Inoculate at 25ᵒC for 4 weeks • Observe hair by making LPCB mount for the presence of conical perforation of hair shaft • Positive - T. mentagrophytes and M. canis
- 46. Hair bait technique • Used to isolate keratinophilic fungi from the soil Technique employed- 1)Place soil on petri dish 2)spread 30 pieces of preferably horse’s hair after sterilization 3)Wet soil with DW and incubate at 30ᵒC 4)When fungal growth appears around hair, 5)transfer a piece of hair on SDA plate. On and under the surface of agar surface. 6)Observe hair under LCB mount.
- 47. Dalmau technique • Stimulation clamydospores formation • Heavy inoculum is streaked and stabbed • Coverslip is placed • Streaking should go beyond coverslip • Incubate for 24-48 hrs at 25ᵒC • Examine at the edge of cover slip (aerobic and max number of clamydospores) • Large refractile thick walled terminal clamydospore • Tryptan blue
- 49. Recent techniques • AccuProbe For identification of fungus ,bacteria, mycobacteria Sensitivity and specificity 100% Detects organisms from primary culture Commercially available probes for identification of cryptococcus spp,histoplasma ,Blastomyces,coccidiodes
- 50. Molecular methods • Hybridization methods • Amplification methods • Sequencing method
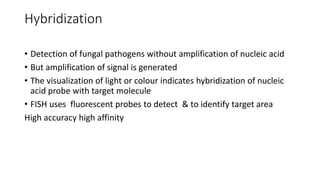
- 51. Hybridization • Detection of fungal pathogens without amplification of nucleic acid • But amplification of signal is generated • The visualization of light or colour indicates hybridization of nucleic acid probe with target molecule • FISH uses fluorescent probes to detect & to identify target area High accuracy high affinity
- 52. PCR • Uses primers to amplify sequence within RNA gene to be amplified • Drawback –do not quantify the amount of amplified DNA (microbial burden) • Rt-PCR - combat this issue of conventional PCR Decreases the time of sample processing (rigid cell wall)
- 53. PCR • Broad range PCR • Nested PCR • Multiplex PCR • Nucleic acid sequence based amplification • Fluorescent Resonance Energy Transfer • TaqMan • Molecular Beacons
- 54. Sequencing • Sanger’s sequencing- selective incorporation of chain terminating dideoxynucelotide by DNA polymerase during invitro DNA replication • Pyrosequencing –determine the order of nucleotide in DNA • Next generation sequencing- better and faster than sanger’s sequencing Detects and identify fungal spp in AD pateints • Ultra deep sequencing – detects organisms with polymicrobial growth Spp of Basidiobolus genus was identified by this method • DNA bar coding – ID using short sections of DNA from standardized genome(super market scanner)
- 55. PNA FISH PNA FISH (Peptide Nucleic Acid Fluorescence In Situ Hybridisation Detects and localizes presence or absence of specific DNA sequence on chromosomes High specificity and sensitivity Used for candida spp
- 56. MALDI-TOF Mass Spectrometry • Rapid ,cost effective, accurate identification of microorganisms • Applied to both mycelial fungi and yeast
- 57. References • Textbook of mycology- Jagdish Chandra • Koneman's Color Atlas and Textbook of Diagnostic Microbiology • Bailey & Scott's Diagnostic Microbiology • Monica Cheesbrough District Laboratory Practice In Tropical Countries Part1