Matter
- 1. Science CHANGE IN MATTER 3rd TERM Boston International School
- 2. ObjectiveObjective To explore the environment to identify matter structure, matter properties and changes, recognizing God as the creator of Matter. ObjectiveObjective To explore the environment to identify matter structure, matter properties and changes, recognizing God as the creator of Matter. Learning objectiveLearning objective Analyzes different elements of the creation identifying matter structure and their properties. Learning objectiveLearning objective Analyzes different elements of the creation identifying matter structure and their properties. Biblical principleBiblical principle Genesis 1:1-2 New King James Version (NKJV) In the beginning God created the heavens and the earth. 2 Now the earth was formless and empty, darkness was over the surface of the deep, and the Spirit of God was hovering over the waters. Biblical principleBiblical principle Genesis 1:1-2 New King James Version (NKJV) In the beginning God created the heavens and the earth. 2 Now the earth was formless and empty, darkness was over the surface of the deep, and the Spirit of God was hovering over the waters.
- 3. “Matter exists in three different states” Matter can exist in three different states, or forms. You can observe matter as a Solid,Solid, LiquidLiquid GasGas “Matter exists in three different states” Matter can exist in three different states, or forms. You can observe matter as a Solid,Solid, LiquidLiquid GasGas
- 4. SOLIDSOLID Definite Shape. Definite Volume. Particles close together, fixed. Little free space between particles Does not flow easily rigid - particles cannot move/slide past one another SOLIDSOLID Definite Shape. Definite Volume. Particles close together, fixed. Little free space between particles Does not flow easily rigid - particles cannot move/slide past one another
- 5. LIQUIDLIQUID Liquids have mass. Liquids take the shape of their container and have definite volume. Liquids take up space. particles can move/slide past one another not easily compressible little free space between particles flows easily LIQUIDLIQUID Liquids have mass. Liquids take the shape of their container and have definite volume. Liquids take up space. particles can move/slide past one another not easily compressible little free space between particles flows easily
- 6. GASGAS particles can move past one another Compressible Lots of free space between particles Flows easily Gases spread out to fill the entire space given and do not have definite volume. Gases have mass. Gases take up space. GASGAS particles can move past one another Compressible Lots of free space between particles Flows easily Gases spread out to fill the entire space given and do not have definite volume. Gases have mass. Gases take up space.
- 10. The 6 Phase Changes 1.Melting: Solid to Liquid 2.Freezing: Liquid to Solid 3.Evaporation: Liquid to Gas 4.Condensation: Gas to Liquid 5.Sublimation: Solid to Gas 6.Deposition: Gas to Solid 7.http://www.chem4kids.com/files/matter_ch anges.html
- 13. Physical properties Physical properties can be observed or measured without changing the identity of the matter. Basically, properties you notice when using one of your five senses: Feel - mass, volume, texture Sight - color Hear Smell Taste
- 15. Physical properties The measurement of mass and other characteristics that can be seen without changing how that object looks are its physical properties. When you look at oranges, you know that they are oranges because of their color, shape, and smell. Mass, color, shape, volume, and density are some physical properties.
- 16. Properties of Matter • A property describes how an object looks, feels, or acts. • The objects shown here have different kinds of properties:
- 18. Physical Changes Changes that do NOT change the identity of the substance. You may or may not be able to undo a physical change.
- 19. Physical Changes: • For example: • 1. Size 2. Shape • 3. State - solid liquid gas • 4. Dilutions • The water doesn't turn into soil or macaroni. • It remains water. • If it did change into soil or macaroni, your drink would taste terrible and you would have an example of a chemical change
- 20. • If you remember, ice is water in the solid state. • When you drop the ice cube into the liquid, it begins to melt because the temperature is higher than that of the ice cube. • It's like putting a snowman on your front lawn in July. • The ice cube becomes liquid water. • This is an example of a physical change. • The solid water turned to liquid water.
- 22. Chemical properties A common chemical property is reactivity. Reactive to oxygen Reactive to air Reactive to water… Notice that chemical propertiesNotice that chemical properties aren’t EASY to observe, unlikearen’t EASY to observe, unlike physical properties.physical properties.
- 23. Chemical Changes Chemical changes do alter the identity of a substance In other words, a chemical change is when something changes into an entirely different substance For example: Iron rusting Wood burning Copper turning to brass Baking a cake spoiled milk
- 24. Chemical properties- • These are properties that can only be observed by changing the identity of the substance. • A piece of paper burns and turns to a black substance. • After the flame goes out you can no longer burn the new substance. • The chemical properties have been changed.
- 25. Milk needs to be in the refrigerator or else it will go bad. If you've ever seen or smelled spoiled milk, it is not a pretty sight. The milk gets a sour odor and becomes lumpy. Unlike physical changes, you cannot reverse chemical changes. You can melt ice to get water and freeze that water to get ice again. You cannot make milk unspoiled.
- 26. Physical vs. Chemical Physical properties: observe without changing the identity of the substance Chemical properties: observe only when the identity changes How do you know if it is chemical or physical? If it CHanges, it’s CHemical
- 28. Measuring Matter Essential ideaEssential idea ““Scientists use tools toScientists use tools to measure the mass and volumemeasure the mass and volume of matter”of matter”
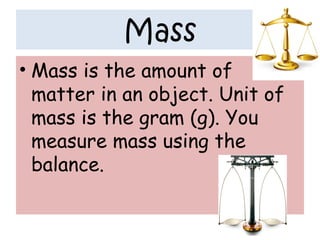
- 29. Mass • Mass is the amount of matter in an object. Unit of mass is the gram (g). You measure mass using the balance.
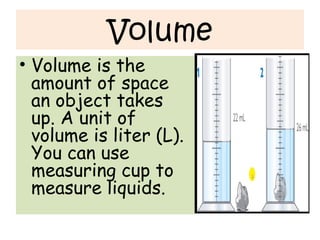
- 30. Volume • Volume is the amount of space an object takes up. A unit of volume is liter (L). You can use measuring cup to measure liquids.
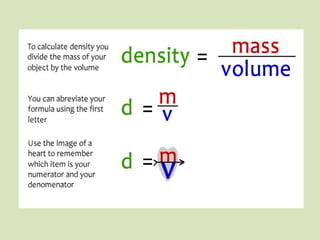
- 31. Density • Density is the amount of mass of a certain volume of an object. Two object can have the same volume but different masses. You have to compare equal volumes of both kinds of matter to compare the density of each object
- 33. Review Questions 1. What state of matter has definite volume and definite shape? 2. Describe the properties of liquids. 3. Describe the differences between gas and liquid. 4. What are physical properties?