Matter (audio) r1
- 1. Structure of Matter What is Matter?
- 2. Everything in the Universe can be classified as either matter or energy! So what is matter? Define matter: Matter is anything that has mass and occupies a volume. Matter is the “stuff” the Universe is made of A Kaboom Key Concept! 1. All matter is composed of very tiny particles This is the first statement of the Particle Model of Matter. 2. Particles are in constant motion. 3. Particles have spaces between them. The spaces are much larger than the particles themselves. 4. Particles are attracted to each other by electrostatic forces. 2
- 3. Particles are in constant motion . . . The energy of the particles in matter determines particle motion and affects the how far apart the particles are from each other. We can think of particle energy as TEMPERATURE. As temperature increases , the average energy of the particles increases The temperature determines the phase or state of the matter 3
- 4. Three Phases of Matter DJY - Chemistry I 4
- 5. Adding heat allows particles to overcome the attractive forces that pull them together. Add heat 5
- 6. Solids – definite volume and shape; particles packed in fixed positions; particles can vibrate back and forth only 6
- 7. Liquids – definite volume but indefinite shape; particles close together but not in fixed positions; particles are free to move (rotate) DJY - Chemistry I 7
- 8. Gases – neither definite volume nor definite shape; particles are at great distances from one another; particles are free to move (translate) DJY - Chemistry I 8
- 9. Plasma – a plasma is an ionized gas. Plasma shares similar characteristics to gases. A plasma is also a very good conductor of electricity and is affected by magnetic fields. 9
- 10. Heating Curve for Water (a pure substance) Water phase changes constant Temperature remains __________ during a phase change. DJY - Chemistry I 10
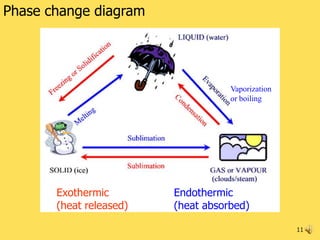
- 11. Phase change diagram Vaporization or boiling Exothermic Endothermic (heat released) (heat absorbed) 11
- 12. Physical changes . . . change a substance WITHOUT changing its chemical makeup. After a physical change, you still have the same substance, it is just in a different form. Examples: boiling, freezing, melting, condensation dissolving split, crack, crush etc 12
- 13. Physical properties .. are qualities which can be observed without changing the substance’s composition. For example: boiling point, freezing point, melting point color hardness density malleability, ductility solubility conductivity 13
- 14. Chemical changes Substances are broken down in chemical reactions and new substances are made. Example - Burning hydrogen (H2) with oxygen (O2) gives H2O. 14
- 15. Chemical Properties describe the how substances react chemically. For example: Metals react in acid to produce H2 gas Zn (s) + HCl (aq) H2 (g) + ZnCl2 (aq) DJY - Chemistry I 15
- 16. In a CHEMICAL CHANGE, the composition of the original substance(s) is altered. The process of chemical change is described as a chemical reaction. chemical change REACTANTS PRODUCTS Different substances are present at the end of the chemical change. The atoms in the reactants are rearranged to make new and different products. 16
- 17. Signs of a Chemical Change Color change Heat Light Gas Produced (not from boiling!) Precipitate – a solid formed by mixing two liquids together DJY - Chemistry I 17
- 18. Physical vs. Chemical Properties Examples melting point flammable density magnetic tarnishes in air 18
- 19. Physical vs. Chemical Properties Examples melting point physical flammable chemical density physical magnetic physical tarnishes in air chemical 19
- 20. Physical vs. Chemical Changes Examples: rusting iron dissolving in water burning wood melting ice grinding spices 20
- 21. Physical vs. Chemical Changes Examples: rusting iron chemical dissolving in water physical burning wood chemical melting ice physical grinding spices physical 21