Organic Compounds
- 1. Chemical and Physical Changes of Organic Compounds 04/30/14 2.5
- 2. Organic Compounds Notes Notes Notes Notes Notes Notes Notes Notes Notes Notes Questions Vocabulary words Formulas Main Ideas Possible Test Questions Key Concepts organic inorganic element compound carbohydrate hydrocarbon protein lipid amino acid nucleic acid DNA Summary of the notes and information learned
- 3. Today’s Warm-up • Let’s review: Physical and Chemical Changes… • BrainPop!
- 4. Physical vs Chemical Change • Physical changes- form or appearance changes but composition stays the same- (Example- phase changes: evaporation, condensation, freezing) • Chemical changes- change the chemical composition of the substance- chemical bonds are broken and reformed. Burning changes wood to ash, smoke, gases • Chemical changes happen at the molecular level, result in new products, and are not easily reversed.
- 5. Physical vs Chemical Change • Changes in matter are constantly occurring around us. • These changes are either physical or chemical • Eating is a great example of both! It involves both physical (chewing with teeth, mixing with tongue) and chemical (breakdown by saliva, digestion by stomach acids) changes.
- 6. Comparing you to the Earth 1 2 3 5 4
- 7. Some basic chemistry… Examples Copper Aluminum Iron Sulfur Water? Salt? Glucose? Why aren’t the last three on the table? They’re compounds! Note the way element names are written…
- 8. Some basic chemistry… Elements vs Compounds
- 9. Compound and Mixtures • Let’s see what Tim and Moby have to say about compounds and mixtures! You should know the definitions for compounds and mixtures and be able explain the differences.
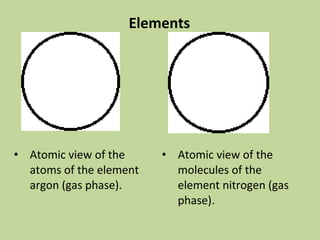
- 11. Elements • Atomic view of the atoms of the element argon (gas phase). • Atomic view of the molecules of the element nitrogen (gas phase).
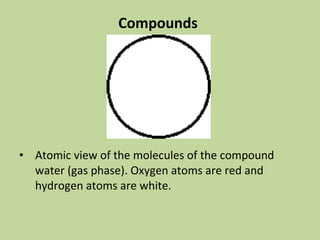
- 12. Compounds • Atomic view of the molecules of the compound water (gas phase). Oxygen atoms are red and hydrogen atoms are white.
- 13. Mixtures Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water).
- 14. All life as we know it is made up of organic compounds.
- 15. Today’s Warmup • What does that word mean- “organic”??? • What does that word mean- “compound”??? • In you lab-book write a definition in your own words. Don’t worry if it turns out to be incorrect or only partially correct, just write what you think.
- 16. Organic compounds always have carbon joined to itself or hydrogen, and other elements like oxygen, and nitrogen, phosphorus, or sulfur.
- 17. There are organic compounds that make up you!- your hair, your skin, even your fingernails… And carbon is a part of all of the compounds.
- 18. So, why is carbon such a special element? https://www.youtube.com/watch?v=ypbb9Zi5Tao Watch a video!
- 19. Virtually every part of our bodies is made with large amounts of this element.
- 21. Why is carbon such a special element? • Each carbon atom can form strong, stable bonds with four other atoms at a time – these are usually oxygen, hydrogen, nitrogen, sulfur, and phosphorus atoms. • Carbon can also bond to other carbon atoms to form chains that are almost unlimited in length. • This creates a huge number and variety of molecules that can be built from carbon atoms. No other element even comes close!
- 22. Imagine carbon as a 4-sided Lego from which you could build a huge variety of things!
- 23. • Virtually every part of your body is made up of these large organic molecules that are based on chains of carbon atoms.
- 24. Chemistry of the Human Body • Let’s see what Tim and Moby have to say about the elements, atoms, and molecules that make up the human body!
- 25. Organic Building Blocks Hydrocarbons are the simplest of the organic compounds. As the name suggests, hydrocarbons are made from hydrogen and carbon. EXAMPLES: CH4
- 26. Organic Building Blocks •The name, carbohydrates, is a good one because it indicates carbon and water (hydrogen and oxygen). •Remember, dehydrated means loss of water, and to be hydrated means to add water. Saltine Mini-Lab!
- 27. Saltine Mini-Lab EXPLORE – Digestion Changes 1. Have students place a soda cracker on a paper towel and crush the cracker with their hand. How did the cracker change? Was this change a physical or chemical change for the cracker? Explain. 2. Have students take the second cracker and chew it for about a minute. Caution students not to swallow the cracker. What was the first taste you noticed in your mouth? What was the second taste you notice?
- 28. Saltine Mini-Lab EXPLORE – Digestion Changes Instructional Note: Students may have to hold the cracker in their mouth for 2 min to allow time for the carbohydrates to break down into sugar. Encourage them to fight the urge to swallow. Do you think this change in taste indicates a physical or chemical change? Soda crackers are high in carbohydrates in the form of starch. An enzyme in saliva begins breaking down the starch into sugar. That is why the cracker tasted sweet. Two forms of digestion occur in the mouth. Physical digestion began when you chewed the cracker. Chemical digestion occurred when the saliva started breaking down the starches.
- 29. Organic Building Blocks •Lipids (oils and fats) are another class of organic compounds built from oxygen, hydrogen, and carbon. It's amazing what these three elements can build!
- 30. Organic Building Blocks Amino acids are the building block for proteins. Proteins are made by connecting amino acids together.
- 31. Organic Building Blocks A few amino acids are built by carbon, hydrogen, oxygen, nitrogen, and sulfur. Mammals need about 20 amino acids to make the proteins they need. Only 2 of these are amino acids containing sulfur.
- 32. Organic Building Blocks We will end our organic building blocks with the ultimate building block of living organisms- DNA. We need just one more element to build it: phosphorus.
- 33. Organic Building Blocks Review • Hydrocarbons= hydrogen + carbon • Carbohydrates and Lipids = hydrogen, carbon, and oxygen • Amino Acids and Proteins = hydrogen, carbon, oxygen, and nitrogen • Some Amino Acids and Proteins = hydrogen, carbon, oxygen, nitrogen, and sulfur • Nucleic Acids = hydrogen, carbon, oxygen, nitrogen, sulfur, and phosphorus
- 34. One more question… • Where do these elements that make up our bodies come from? I mean, where did the come from originally??? • They came from exploding stars! – But that’s a lesson for another day…
Editor's Notes
- http://periodictable.com/Elements/006/index.html
- http://periodictable.com/Elements/006/index.html